YANINA CASTILLO COSTA, VÍCTOR M. MAURO, MAURO GARCÍA AURELIO, CARLOS BARRERO,
ADRIÁN CHARASK, JUAN A. GAGLIARDI
Área de Investigación y Consejo de Emergencias Cardiovasculares,
Sociedad Argentina de Cardiología (SAC)
Abstract Cardiogenic shock (CS) in the setting of an ST-segment elevation myocardial infarction (STEMI) is
a severe complication and constitutes one of the principal causes of death associated with this condition. The aim of this study was to describe the clinical characteristics, treatment strategies and hospital outcome of CS associated with STEMI in Argentina. The Argentine Registry of Cardiogenic Shock (ReNA-Shock) was a prospective and multicenter registry of consecutive patients with CS hospitalized in 64 centers in Argentina between July 2013 and May 2015. Only those with ST-segment elevation myocardial infarction (STEMI) were selected for this analysis. Of the 165 patients included in the ReNa-Shock registry, 124 presented STEMI. Median age was 64 years (IQR 25-75: 56.5-75) and 67% were men; median time from symptom onset to admission was 240 minutes (IQR 25-75: 132-720). 63% of the cases presented CS at admission. Eighty-seven percent underwent reperfusion therapy: 80% primary percutaneous intervention with a median door-to-balloon time of 110 minutes (IQR 25-75: 62-184). Inotropic agents were used in 96%; 79% required mechanical ventilation; a Swan Ganz catheter was inserted in 47% and 35% required intra-aortic balloon pumping. Most patients (59%) presented multivessel disease (MV). Hospital mortality was 54%. Multivariate analysis identified that time from symptom onset to admission (> 240 min) was the only independent predictor of mortality (OR: 3.04; CI 95%: 1.18-7.9). Despite using treatment strategies currently available, morbidity and mortality of STEMI complicated with CS remains high.
Key words: cardiogenic shock, myocardial infarction, registries
Resumen Shock cardiogénico en el síndrome coronario agudo con elevación del ST. Resultados del
ReNa-Shock ST. El shock cardiogénico (SC) en el síndrome coronario agudo con elevación del ST (SCACEST), es una complicación grave y constituye una de las principales causas de muerte. El objetivo del registro fue conocer las características clínicas, estrategias de tratamiento y evolución intrahospitalaria del SC secundario a un SCACEST en Argentina. El Registro Argentino de Shock Cardiogénico (ReNa-Shock) fue prospectivo, multicéntrico y consecutivo de pacientes internados con SC en el periodo 2013/2015 en 64 centros de Argentina. Fueron incluidos 165 pacientes, de los cuales124 presentaban SCACEST. La edad (mediana) fue de 64 [RIC25-75: 56-75] años, 67% hombres. La mediana de tiempo desde el inicio de los síntomas al ingreso hospitalario fue de 240 minutos [RIC25-75: 132-720]. Un 63% de los casos tuvo SC desde el ingreso. El 87% recibió tratamiento de reperfusión, con un 80% de angioplastia primaria y un tiempo puerta-balón (mediana): 110 minutos [RIC25-75: 62-184]. Requirieron inotrópicos un 96%, asistencia respiratoria mecánica el 79%, catéter de Swan Ganz 47%, balón de contrapulsación intraaórtico 35%. El 59% tenía lesión de 2 o 3 vasos. La mortalidad intrahospitalaria fue 54%. En el análisis multivariado, solo el tiempo de evolución al ingreso (345 min [RIC25-75: 135-720] vs. 180 min [RIC25-75: 85-420]; p: 0.03) fue la única variable predictora independiente de mortalidad. La morbimortalidad del SC complicando un SCACEST es elevada a pesar de la utilización de las estrategias de tratamiento actualmente disponibles.
Palabras clave: shock cardiogénico, infarto de miocardio, registro
Received: 9-IX-2016 Accepted: 20-IV-2017
Postal address: Yanina Castillo Costa, Azcuénaga 980, 1425 Buenos Aires, Argentina
e-mail: yanu_c@hotmail.com
Cardiogenic shock (CS) is not a frequent condition, but constitutes the leading cause of death in patients hospitalized with acute myocardial infarction. Its incidence ranges from 6% to 8% and is associated with a mortality rate of 40-50% despite myocardial revascularization and the use of intra-aortic balloon pumping (IABP)1, 2. The information currently available comes from studies and registries performed more than 10 years ago3, 4. In Argentina, the information is scarce and derived from registries of acute coronary syndromes performed by the Argentine Society of Cardiology (SAC)5, 6.
The National Registry of Cardiogenic Shock (Registro Nacional de Shock Cardiogénico, Re-Na-Shock) was the first registry performed in Argentina7 specially designed to determine the clinical characteristics, treatment strategies, and in-hospital events in patients admitted to the critical care units with ACS with or without ST-segment elevation and who present CS at the moment of admission or during hospitalization. This paper presents the data of cases with CS associated with ST-segment elevation myocardial infarction (STEMI)7.
Materials and methods
Re-Na Shock patients with STEMI complicated with CS admitted to coronary care units from July 2013 to May 2015 were entered in the present analysis. Cardiogenic shock was defined as systolic blood pressure ≤ 90 mmHg for at least 30 minutes or requiring vasopressors or inotropic drugs to maintain a systolic blood pressure ≥ 90 mmHg, associated with clinical signs of hypoperfusion or pulmonary congestion in the absence of hypovolemia or arrhythmias which could account for the clinical condition8.
Data were collected by the investigators of the different centers and entered in an ad hoc designed electronic worksheet containing the following variables: age, gender, risk factors, comorbidities, previous treatment, infarct location, Killip class, time from symptom onset to admission, reperfusion strategies (thrombolysis or angioplasty), number of vessels involved (coronary artery stenosis ≥ 70% or occlusion) and treated, drug therapy, invasive monitoring with a pulmonary artery catheter, mechanical support with intra-aortic balloon pump (IABP) and mechanical ventilation. Hospital outcome and complications (fever, sepsis, multiorgan failure, arrhythmias, angina, reinfarction, requirement of red blood cell transfusions and major or minor bleeding) were also recorded. Echocardiographic data and lab tests at admission and at 24 hours were analyzed. Severe bleeding was defined using the TIMI bleeding criteria of major bleeding9 or the GUSTO scale of moderate or severe bleeding10.
The protocol was organized and conducted by the SAC’s Research Department and the Council of Cardiovascular Emergency Care and was approved by the SAC’s Bioethics Committee.
A frequency table was constructed using all the variables observed. Continuous variables with normal and non-Gaussian distribution were presented as mean ± standard deviation, or median and interquartile range (IQR 25-75) respectively and were compared using the Student’s t test or the Wilcoxon rank sum test as applicable. Discrete variables were expressed as percentages and were compared using the chi-square test with the Yates correction or the Fisher’s exact test as applicable.
Contingency table analysis was used to compare the association or the independence of the variables. The presence of associations or independent predictions between the different variables involved and mortality was determined using multiple logistical regression analysis. Those variables with a p value = 0.10 at univariate analysis were included in the different regression models. The value corresponding to each covariate was expressed as adjusted odds ratio with its corresponding 95% confidence interval. A two-tailed p value < 0.05 was considered statistically significant.
Results
Sixty-four critical care units nationwide participated in the study (74% coronary care units, 17% intensive care units and 9% polyvalent intensive care units). The registry included 165 patients, 124 (75%) were STEMI. The clinical characteristics are summarized in Table 1. Median time from symptom onset to admission was 240 minutes (IQR 25-75: 132-720) and 87% underwent coronary artery reperfusion: 80% primary PCI, 20% thrombolytic therapy (83% streptokinase) and 13% rescue PCI. Eighty percent of the procedures were successful, with a median door-to-balloon-time of 110 minutes (IQR 25-75: 62-184). Sixteen patients did not undergo reperfusion due to late hospital arrival (9 cases), unavailable reperfusion therapy (1 case) and non-reported causes in 6 patients.
During hospitalization, 87% of patients underwent coronary angiography. Most (59%) presented MV disease (28% two-vessel disease and 31% three-vessel disease). In 32% of MV disease patients, non-culprit vessels were also intervened, in most cases (95%) during the same procedure. Sixty-three percent presented CS at admission and 19% of the remaining patients were admitted in Killip class I, 12% in class II and 6% in class III and developed CS at a median time of 7 h (IQR 25-75: 1.2-28) after hospitalization. Finally, 14% presented CS after 24 hours of admission.
Inotropic agents or vasoactive drugs were used in 96% of the cases (norepinephrine 75%, dopamine 54%, dobutamine 56% and levosimendan 5%); 79% required mechanical ventilation and IABP was used in 35% of cases for a median of 3 days (IQR 25-75: 1-4.5). The complications associated with IABP occurred in 16%: stroke (n = 1); acute lower limb ischemia (n = 2), thrombocytopenia (n = 4) and severe bleeding (n = 1).
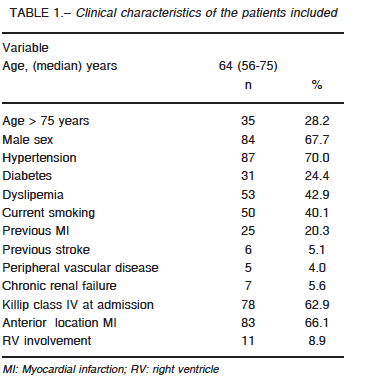
A Swan-Ganz (SG) catheter was inserted in 47% of the treated: 62% within the first day, 18% between 24 and 48 hours and 20% after 48 hours for a median of 3 days (IQR: 2-5). The catheter was inserted as a routine practice or to guide treatment in most cases, and for diagnostic purposes in 15%. There were no differences in age, gender, infarct location, time from symptom onset to admission, type of reperfusion strategy or right ventricle involvement between patients with a SG catheter inserted and those without.
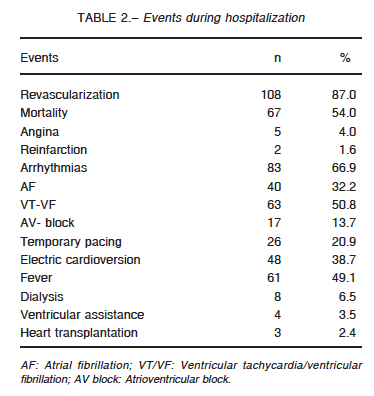
Main events during hospitalization are presented in Table 2.
The incidence of bleeding was 11% and it was severe in 7 patients. Twenty-one percent required transfusion of red blood cells (< 2 units: 23%, 2 to 4 units: 54% and > 4 units: 23%). Twenty-nine patients were successfully resuscitated and 52% survived and were discharged.
The echocardiographic data were analyzed in 96 cases. Forty-seven percent of the echocardiograms were performed within 24 hours of admission. Echocardiography was not performed in 23% (70% of these patients had died within the first 48 h of admission).
Ventricular function was visually estimated by echocardiography: 58% evidenced severe and 29% moderate dysfunction, while the remaining 21% had normal or near normal of left ventricular function. Wall motion abnormalities corresponded to a single vascular territory in 38% of the cases and to 2 or more in 62%. The characteristics of wall motion in remote territories were reported in 70 patients: 54% presented a preserved wall motion, 41% abnormal and 4% evidenced hyperkinesis. Systolic pressure in the pulmonary artery could be measured in 31 patients with a median value of 39 mm Hg (IQR 25-75: 22-48). Five patients presented mitral regurgitation and 1 had a ventricular septal defect.
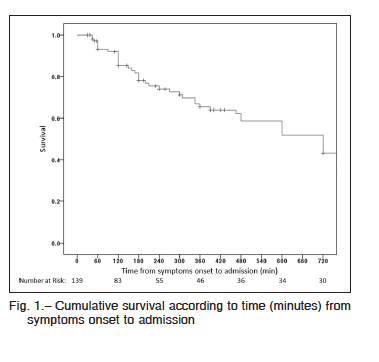
Hospital mortality was 54% (46% of them within the first 48 h). The most frequent causes of death were ventricular failure (53%), arrhythmias (25%), infections (12%) and neurological complications (4%). Mortality rate was 51% in reperfused patients and 75% in those without reperfusion (p = 0.05), 37% in patients with one-vessel disease versus 54% in those with MV disease (p = 0.05). In patients undergoing MV PCI, mortality was 67% while in those with MV disease who underwent PCI of the culprit vessel mortality was 36% (p = 0.01). There were no differences in mortality according to the use of IABP. On the other hand, mortality was significantly higher in those who were not monitored with a SG catheter (55% vs. 45%; p = 0.004).Cumulative survival was 0.85 for patients lasting 120 minutes from symptom onset to admission and 0.74 when it was 240 minutes (Fig. 1).
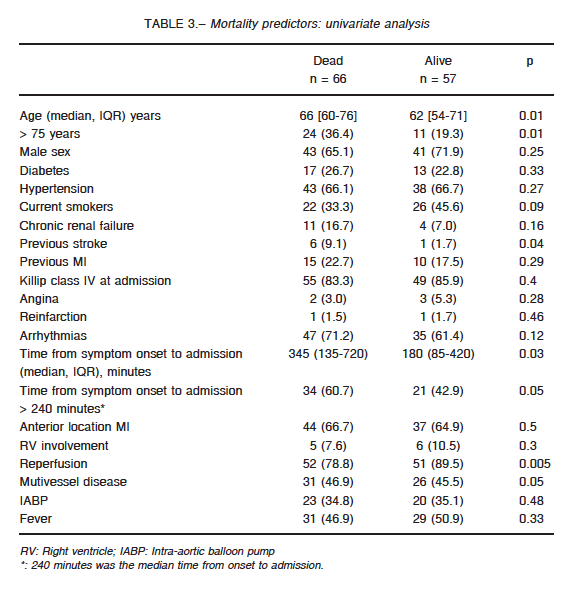
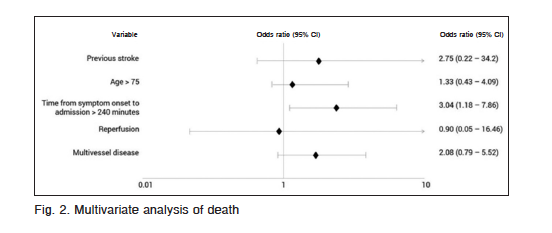
At univariate analysis, age > 75 years, previous stroke, time from symptom onset to admission, reperfusion and multivessel disease were associated with higher mortality (Table 3). At multivariate analysis time from symptom onset to admission > 240 minutes, was the only independent predictor of mortality (OR: 3.04; CI 95%: 1.18-7.9; p = 0.021) (Fig. 2).
Discussion
Cardiogenic shock is the most life-threatening complication of MI and remains the leading cause of death. Historically, the incidence of CS ranged between 5 to 15% but many registries have reported that the implementation of reperfusion strategies reduced the incidence of this complication11-13. In Argentina, CS incidence is 6% according to the 2011 registry of the Sociedad Argentina
de Cardiología; when data from the same centers were compared in different time intervals from 2001 to 2005 the incidence decreased from 12% to 5%5.
In our registry and in line with other reports, most CS admitted were due to STEMI14. Also, the prevalence of males with CS was greater than that of females but the relative proportion of women was greater than that of men´s when compared with populations with ACS without shock in accordance with the reports of all the registries available3, 4, 15, 16.
The age of our population was similar to that of international registries11. In the setting of an ACS, CS may be present at hospital admission (< 20% of the cases)3, 4, or may develop during hospitalization. In our registry, 63% of the patients presented CS from admission and the rest of the patients developed CS at a median of 7 hours from admission, data comparable to other reported findings2, 3, 17.The different prevalence of CS at the time of hospital admission in the present study compared to other registries may be related to a longer time from symptom onset
to first medical contact (6 h vs. 1.5 h). Nevertheless, the majority of our patients with CS were admitted during the first day of symptoms similar to data published in previous registries3, 4.
One of the benefits of reperfusion therapy is to reduce the incidence of CS by limiting infarct size18, 19. The high mortality rate of MI patients complicated with CS and the results of the SHOCK trial3 as regards reduction of mortality with an early reperfusion therapy have motivated the American20, European21 and local22 guidelines to strongly recommend prompt revascularization in patients with CS. Moreover, a complete revascularization in cases of multivessel disease that can jeopardize remote left ventricle wall motion is recommended despite the lack of strong evidence warranting this indication. Multivessel disease is very frequent in patients with CS: 64% in our study and between 60% and 78% in reported evidence4, 23.
Despite the recommendations of the guidelines, the single culprit vessel is treated in most CS patients. Similarly to other registries23, the non-culprit vessels were treated in only one third of the patients with MV disease in our study but with a higher mortality compared with patients who underwent single revascularization of the culprit vessel. In addition, the results of the German ALKK-PCI registry that have been recently published24, reported that patients undergoing immediate multivessel PCI evidenced an increased mortality compared with patients undergoing single culprit vessel revascularization (47% vs. 36%) that persisted after multivariate analysis. Yet, data registry must be interpreted with caution as patients with a more extensive coronary disease could have received a complete revascularization and thus the higher mortality in this group can be attributed to a higher clinical risk rather than to the revascularization procedure per se. The ongoing CULPRIT-SHOCK trial25 will address the question of the optimal revascularization strategy in CS patients with multivessel disease.
The use of IABP was not associated with differences in mortality rate. Despite IABP has been a mainstay of treatment of CS since it was introduced in clinical practice in 1960 and was recommended by the American and European guidelines as a class I indication, its use in clinical practice varies between 15% and 40%11. An analysis of the National Registry of Myocardial Infarction in the United States26 showed that the use of IABP decreased from 36.5% in 1998 to 13.4% in 2008, and this reduction is similar to reports from other registries12. Despite the theoretical virtues of the device in the management of CS, the IABP-SHOCK II trial27 which is the only randomized trial to address this issue so far, could not demonstrate significant differences in mortality of patients treated with IABP and thus its use is currently a class II recommendation21, 28 and even a class III in the last guideline published29.
The use of other ventricular assist devices was 2.4% in our study and similar to previous reports15. Probably, the use of other ventricular assist devices (ECMO, Impella) may increase in the near future, as many studies have reported a better clinical outcome associated with their use30, 31.
Although a few studies have reported a decline in mortality associated with CS throughout the years8, 32, it still remains very high with differences related to variables such as age, gender33, extension of coronary artery disease, angiographic patency, successful revascularization, type of infarction and early or late CS development14.
Most reports of CS evidenced a mortality ranging from 40 to 60%12, 34, 35, in line with the one observed in our study that was 54%. Finally, the time from the onset of symptoms to admission was the only independent predictor of mortality in our population.
The limitation of this study is that the present registry represents the real treatment of CS associated with STEMI in Argentina in patients mostly recruited in tertiary care centers; thus, these results cannot be extrapolated to CS at admission or developed during hospitalization in other type of centers.
Finally, the characteristics of CS in Argentina are similar to those of populations worldwide. In our registry, CS was more frequent at admission, probably related to longer time delays from symptom onset to first medical contact. Only one third of our patients with mutivessel disease underwent a complete revascularization procedure with a high mortality despite all the treatment strategies available.
Conflict of interests: None to declare
-
References
1. Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005; 294: 448-54.
2. Menon V, Hochman JS, Stebbins A, et al. Lack of progress in cardiogenic shock: lessons from the GUSTO trials. Eur Heart J 2000; 21: 1928-36.
3. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock? N Engl J Med 1999; 341: 625-34.
4. Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction-etiologies, management and outcome: a report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries for cardiogenic shock? J Am Coll Cardiol 2000; 36: 1063-70.
5. Garcia Aurelio M, Cohen Arazi H, Higa C, et al. Infarto agudo de miocardio con supradesnivel persistente del segmento ST. Registro multicéntrico SCAR (Síndromes Coronarios Agudos en Argentina) de la Sociedad Argentina de Cardiología. Rev Argent Cardiol 2014; 82: 259-67.
6. Gagliardi JA, Charask A, Higa C, et al. Infarto agudo de miocardio en la Republica Argentina. Análisis comparativo en los últimos 18 años. Resultados de las Encuestas SAC. Rev Argent Cardiol 2007; 75: 171-8.
7. Castillo Costa Y, Garcia Aurelio M, Mauro V, et al. Argentine National Registry of Cardiogenic Shock (ReNa-SHOCK). Rev Argent Cardiol 2016; 84: 221-7.
8. Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 2009; 119: 1211-9.
9. Antman EM, Morrow DA, McCabe CH, et al. Enoxaparin versus unfractionated heparin as antithrombin therapy in patients receiving fibrinolysis for ST-elevation myocardial infarction. Design and rationale for the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment-Thrombolysis in Myocardial Infarction Study 25 (ExTRACT-TIMI 25). Am Heart J 2005; 149: 217-26.
10. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med 1993; 329: 673-82.
11. Movahed MR, Khan MF, Hashemzadeh M, Hashemzadeh M. Age adjusted nationwide trends in the incidence of all cause and ST elevation myocardial infarction associated cardiogenic shock based on gender and race in the United States. Cardiovasc Revasc Med 2015; 16: 2-5.
12. Redfors B, Angeras O, Ramunddal T, et al. 17-year trends in incidence and prognosis of cardiogenic shock in patients with acute myocardial infarction in western Sweden. Int J Cardiol 2015; 185: 256-62.
13. Awad HH, Anderson FA, Jr., Gore JM, Goodman SG, Goldberg RJ. Cardiogenic shock complicating acute coronary syndromes: insights from the Global Registry of Acute Coronary Events. Am Heart J 2012; 163: 963-71.
14. Holmes DR, Jr., Berger PB, Hochman JS, et al. Cardiogenic shock in patients with acute ischemic syndromes with and without ST-segment elevation. Circulation 1999; 100: 2067-73.
15. Kunadian V, Qiu W, Ludman P, et al. Outcomes in patients with cardiogenic shock following percutaneous coronary intervention in the contemporary era: an analysis from the BCIS database (British Cardiovascular Intervention Society). JACC Cardiovasc Interv 2014; 7: 1374-85.
16. Aissaoui N, Puymirat E, Tabone X, et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J 2012; 33: 2535-43.
17. Webb JG, Sleeper LA, Buller CE, et al. Implications of the timing of onset of cardiogenic shock after acute myocardial infarction: a report from the SHOCK Trial Registry. Should we emergently revascularize Occluded Coronaries for cardiogenic shock? J Am Coll Cardiol 2000; 36: 1084-90.
18. Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI). Lancet 1986; 1: 397-402.
19. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994; 343: 311-22.
20. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362-425.
21. Task Force on the management of STseamiotESoC, Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33: 2569-619.
22. Consenso de Síndromes Coronarios Agudos con Elevación del Segmento ST. Sociedad Argentina de Cardiología. Rev Argent Cardiol 2015; 82: 1-47.
23. Trzeciak P, Gierlotka M, Gasior M, et al. Mortality of patients with ST-segment elevation myocardial infarction and cardiogenic shock treated by PCI is correlated to the infarct-related artery–results from the PL-ACS Registry. Int J Cardiol 2013; 166: 193-7.
24. Zeymer U, Hochadel M, Thiele H, et al. Immediate multivessel percutaneous coronary intervention versus culprit lesion intervention in patients with acute myocardial infarction complicated by cardiogenic shock: results of the ALKK-PCI registry. EuroIntervention 2015; 11: 280-5.
25. Thiele H, Desch S, Piek JJ, et al. Multivessel versus culprit lesion only percutaneous revascularization plus potential staged revascularization in patients with acute myocardial infarction complicated by cardiogenic shock: Design and rationale of CULPRIT-SHOCK trial. Am Heart J 2016; 172: 160-9.
26. Patel H, Shivaraju A, Fonarow GC, et al. Temporal trends in the use of intraaortic balloon pump associated with percutaneous coronary intervention in the United States, 1998-2008. Am Heart J 2014; 168: 363-73 e12.
27. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287-96.
28. American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American College of Emergency P, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61: e78-140.
29. Authors/Task Force, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35: 2541-619.
30. Lawler PR, Silver DA, Scirica BM, Couper GS, Weinhouse GL, Camp PC, Jr. Extracorporeal membrane oxygenation in adults with cardiogenic shock. Circulation 2015; 131: 676-80.
31. O’Neill WW, Schreiber T, Wohns DH, et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol 2014; 27: 1-11.
32. Carnendran L, Abboud R, Sleeper LA, et al. Trends in cardiogenic shock: report from the SHOCK Study. Should we emergently revascularize occluded coronaries for cardiogenic shocK? Eur Heart J 2001; 22: 472-8.
33. Fengler K, Fuernau G, Desch S, et al. Gender differences in patients with cardiogenic shock complicating myocardial infarction: a substudy of the IABP-SHOCK II-trial. Clin Res Cardiol 2015; 104: 71-8.
34. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol 2004; 44: E1-E211.
35. Van de Werf F, Ardissino D, Betriu A, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2003; 24: 28-66.
