FABIO A. PERSIA ¹, ESTEFANÍA RINALDINI ¹, ADRIANA CARRIÓN ²,
MARÍA BELÉN HAPON ¹, ³, CARLOS GAMARRA-LUQUES ¹, ²
¹ Instituto de Medicina y Biología Experimental de Cuyo – CONICET,² Facultad de Ciencias Médicas, Universidad Nacional de Cuyo,³ Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Cuyo, Mendoza, Argentina
Abstract Higher plants have provided various natural derived drugs used currently in western medicine.
Tessaria absinthioides (Hook. & Arn.) DC, Asteraceae, is a native plant from South-America with reported ethnopharmacological and culinary uses. Despite recent scientific reports about plants properties, there is not a well conducted research about its anticancer and potential toxic effects. The current work demonstrates the plant aqueous extract composition; the in vitro induced cytotoxicity, and explores, in vivo, its oral toxicity and antitumoral effects. Composition of aqueous extract was determined by phytochemical reactions. Cytotoxicity was tested in tumoral (Hela, Gli-37, HCT-116 and MCF-7) and non-tumoral (HBL-100) cells, using MTT assay. Oral toxicity and the antitumor activity against colorectal carcinoma were studied in rodents. The chemical analysis revealed the presence of flavonoids, carbohydrates, sterols, terpenes and tannins. Cytotoxicity towards tumoral cells was observed (CV50: 3.0 to 14.8 μg/ml); while in non-tumoral cells, extracts evidenced a selective reduced toxicity (CV50: 29.5 μg/ml). Oral administration of the extract does not induce acute nor dose-repeated toxicity at doses up to 2000 mg/kg and 1000 mg/kg/day, respectively. The antitumoral effect was confirmed by a significant increase in a median survival from 24 weeks (non-treated) to 30 weeks (T. absinthioides treated). The present data indicate that T. absinthioides extract exhibits cytotoxicity against cancer cell lines, with no-toxic effects and significant antitumoral effects in colorectal cancer when is orally administrated. In conclusion, T. absinthioides possesses selective cytotoxicity and antitumoral activities, making its plant derivatives products promising for cancer research and treatment.
Key words: toxicity, phytomedicine, oncology, native plants
Resumen Evaluación de las propiedades citotóxicas y antitumorales del extracto acuoso de Tessaria
absinthioides (Hook & Arn) DC, “pájaro bobo”. Las plantas superiores han provisto numerosos derivados naturales usados actualmente por la medicina occidental. Tessaria absinthioides (Hook & Arn) DC, Asteraceae, es una planta autóctona de Sudamérica con informes de uso etnofarmacológico y culinario. A pesar de los reportes científicos sobre las propiedades de esta planta, no existen estudios que caractericen sus efectos antitumorales ni sus efectos tóxicos. En el presente trabajo se describe la composición del extracto acuoso de T. absinthioides, sus propiedades citotóxicas in vitro, y explora in vivo la toxicidad oral y su capacidad de afectar la progresión de tumores. La composición se determinó mediante reacciones fitoquímicas. La citotoxicidad se estudió en líneas celulares tumorales (Gli-37, HeLa, HCT-116 y MCF-7) y no tumorales (HBL-100), utilizando el ensayo de MTT. La toxicidad oral de los extractos y su capacidad antitumoral sobre carcinoma colorrectal se analizaron en roedores. El análisis del extracto acuoso evidenció flavonoides, carbohidratos, esteroles, terpenos y taninos. La citotoxicidad sobre células tumorales resultó similar a la observada para el 5-fluoracilo (CV50: 3.0 a 14.8 μg/ml); mientras que, en células no tumorales, el efecto estuvo selectivamente reducido (CV50: 29.5 μg/ml). La administración oral del extracto no indujo toxicidad aguda ni a dosis repetidas (dosis hasta 2000 mg/kg y 1000 mg/kg/día, respectivamente). Los efectos antitumorales se confirmaron mediante un significativo aumento de la supervivencia en el grupo tratado con T. absinthioides. En conclusión, de acuerdo a los resultados obtenidos, T. absinthioides y sus derivados naturales representan un campo prometedor de estudio para la investigación en el tratamiento del cáncer.
Palabras clave: toxicidad, fitomedicina, oncología, plantas autóctonas
Received: 4-X-2016 Accepted: 4-V-2017
Postal address: Carlos Gamarra-Luques, Instituto de Medicina y Biología Experimental de Cuyo – CCT Mendoza, Av. Ruiz Leal s/n, Casilla de Correo 0855, 5500 Mendoza, Argentina
e-mail: cgamarraluques@gmail.com
Along with compounds from terrestrial microorganisms, the constituents of higher plants have provided a substantial number of the natural product-derived drugs used currently in western medicine1. Elucidation of bioactive plants derived compounds is an expanding field of research, particularly of species that are used in systems of traditional medicine or are utilized as botanical dietary supplements.
Tessaria absinthioides (Hook. & Arn.) DC from the family Asteraceae, is currently named ‘pájaro bobo’, ‘suncho negro’, or ‘brea’. It is a frequent species inhabiting sandy and wet soils in Bolivia, Chile, Uruguay and Argentina, where it covers thousands of square miles in the river basins2. It is an aromatic plant commonly used in traditional medicine and as spices or seasonings through Argentina and Chile territories3. Its infusions have been employed since Aymara and Huarpe folk Medicine´s as an hypocholesterolemic, balsamic, expectorant, for hepatitis, renal insufficiency, diabetes and “empacho” (digestive disorder with a halt of undigested food and non-digestible substances, provoking a stagnation of intestinal transit), along both countries4-6.
The potential therapeutic use of this plant derivatives, have conducted to the isolation of pharmacologically active components with specific biological activities. The aqueous and organic extracts and its purified essential oils have demonstrated virucidal activity against herpes simplex virus type 1 and 2, poliovirus type 2; and Junin virus7. The hidroalcoholic extract, constituted by coumarins, flavonoids, tannins and saponins, have been related to anti-inflammatory effects by inhibition of pro-inflammatory enzymes8. Insecticidal effects have been related to isolated sesquiterpenes which induce topical toxicity, growth alteration effects and repellent activity to Tenebrio molitor and Tribolium castaneum larvae, a serious stored-product insect pest with a world–wide distribution9, 10. Furthermore, isolated tessaric acid has shown in vivo gastric cytoprotective activity11.
Despite recent scientific reports about the plant derived compounds identification and activities, in accordance to our knowledge, there is not a well conducted research about its antitumoral properties and potential toxic effects in animal models. In consequence, the present work aims were: to determine by phytochemical qualitative analysis the composition of T. absinthioides aqueous extract; to study the extract in vitro activity against tumoral and non tumoral cell lines; to discard toxicity induced by its oral administration in laboratory healthy animals; and to show the extract capability to affect in vivo tumor induction. The results of this work are compared with the chemotherapeutic agent 5-fluoracil (5-FU), which have well characterized antitumoral and toxic effects.
Materials and methods
Tessaria absinthioides (Hook & Arn) DC, was collected in Lavalle-County, Mendoza-Argentina (33°44’10”S, 68°21’30.5”W). A voucher specimen (MERL-61823) was deposited in the Mendoza Ruiz Leal herbarium. Leaves were obtained immediately. Then, 20 g of leaves were autoclaved in 200 ml of distilled water. Solids were separated by filtration and the obtained volume was lyophilized. At the moment of use, lyophilized was reconstituted with milliQ water (1 g/ml) and sterilized by passing through a 0.22 µm pore size filter.
A qualitative phytochemical screening for chemical constituents was realized on the aqueous extract. Alkaloids12, 13, flavonoids14, resins15, 16, carbohydrates17, amino acids18, sterols/terpenes19, and tannins20 were evidenced by colorimetric, and precipitation reactions. Identified chemical constituents were extracted by solid-liquid procedures from 1 g of lyophilized crude extract by selective solvents (HPLC grade – J.T Baker), dried obtained compounds were quantified.
In vitro studies were realized in the human cell lines: HeLa, uterine cervix adenocarcinoma; Gli-36, glioblastoma; HCT-116, colorectal carcinoma; MCF-7, breast adenocarcinoma; and HBL-100, non-tumoral epithelium (American Type Culture Collection, ATCC); and cultured in DMEM, supplemented with 10% fetal bovine serum, 100 IU of penicillin and 100 μg/ml streptomycin (GIBCO – USA). Culture conditions were fixed at 37 °C, in a humidified atmosphere enriched by 5% CO2.
The cytotoxic activity was studied by MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide)] assay. This process requires active mitochondria, and the amount of MTT cleaved is directly proportional to the number of viable cells present. Cells were seeded in 96-well microplates; 24 hours later, the medium was aspirated and replaced by medium containing T. absinthioides (0 to 50 μg/ml) or 5-FU (Filaxis®, Argentina) at indicated doses (0 to 50 μg/ml). Then, cells were incubated for 72 hours; thereafter, medium was replaced by 100 μl of MTT solution (0.5 mg/ml in DMEM, without phenol red nor FBS) and incubated for an additional 4 h. MTT solution was then removed and 100 μl of DMSO added; the plates were shaken for 10 min to dissolve the formazan crystals. The optical density was measured using a ThermoScientific Multiscan Elisa reader at 570 nm. The optical density obtained in untreated cells was taken as 100% viability and the other values were calculated in accordance with controls (concentration 0 µg/ml). The assay was performed three times in triplicate. To quantify the measured cytotoxicity, the 50% of cell viability (CV50) was used.
The animals used in this work were cared in accordance with the Guiding Principles in the Care and Use of Animals of the US National Institute of Health. All procedures were approved by the Institutional Animal Care and Use Committee of School of Medical Science, Universidad Nacional de Cuyo (Protocol approval N°30/2014).
To perform the in vivo toxicity studies, Sprague Dawley rats bred in our laboratory, 8 weeks-old at the onset of treatment were used. They were kept in a controlled light (lights on 6:00 AM to 10:00 PM) and temperature (22-24 °C) room. Rats chow and tap water were available ad libitum.
The acute toxicity study procedure was performed according to the guideline for the testing of chemicals, acute oral toxicity (OECD 200121). A single dose of aqueous extract, was administered by oral gavage to 4 groups of 3 female and 3 male rats each, at doses of 0 (described as control group), 50, 300, or 2000 mg/kg, respectively. The rats were monitored for mortality, body weight changes and other clinical signs during 14 days after dosing. All rats were necropsied to detect grossly observable evidence of organ or tissue damage or dysfunction.
The 28-day repeated-dose study was performed according to the guideline for the testing of chemicals, repeated dose 28-day oral toxicity study in rodents (OECD 200822). Four groups of 3 female and 3 male rats each were administered daily, for 28 days, by oral gavage doses of aqueous extract of T. absinthioides. The doses were 0 (described as control group), 250, 500 and 1000 mg/kg/day. Body weights, water consumption, and general clinical observations were evaluated three times a week. All animals were evaluated daily for abnormal behavior and presence of clinical symptoms considered as endpoints. Observation time was extended until 1 day after the exposure period. Then, animals were sacrificed and given a gross and microscopic pathology examination. Troncal blood was collected; one aliquot was obtained with 10% EDTA and used for blood cells quantification. The other aliquot was collected to serum separation and then used for biochemical determinations.
The in vivo antitumor activity of aqueous extract was studied in adult male BALB/c mice, bred in our laboratory, 6 weeks old at the onset of treatment. They were kept in a light (lights on 6:00 AM to 10:00 PM) and temperature (22-24 °C) room. Mice chow (Cargill, Córdoba, Argentina) and tap water were available ad libitum. Four groups of 8 animals each were separated. Group 1, did not receive treatments and was considered as survival control. In the other 3 groups, colorectal tumor induction was performed by DMH, 20 mg/kg/week, for 22 weeks, S.C.22 Group 2, animals were tumor inducted and did not receive other treatment, it was considered as indicative of no treated tumor survival. Group 3, 8 weeks after tumor induction, animals receive 5-FU 30 mg/kg/week, I.P.23, until animal sacrifice. Finally (group 4), 8 weeks after tumor induction, animals were treated with a dilution of lyophilized T. absinthioides in the drinking water. Mice extract consumption was 300 mg/animal/day, until animal sacrifice. T. absinthioides dose was selected as a higher concentration of extract that not affected the animal water consumption. The human endpoints to sacrifice animals were: reduction of spontaneous activity, respiratory difficulty, not eating/drinking, increased/decreased weight more than 20%, diarrhea, no micturition, anal bleeding and anal tumor protrusion.
Along the work, data are expressed as mean ± standard error (SEM) and analyzed using GraphPad Prism 5.0 software. To assess the 50% of cell viability (CV50), a sigmoidal dose-response analysis was performed; values were considered acceptable when goodness of fit showed R2 ≥ 0.90. Comparisons between two groups were done using Student’s T test. When more than two groups were compared, one-way ANOVA followed by Dunnett’s multiple comparison test was used. Animal survival curves were compared using a Mantel Cox test. In all cases, statistical significance was considered when p < 0.05.
Results
The qualitative phytochemical analysis of the aqueous extract revealed the presence of different chemical groups. Strong positive reactions were registered for flavonoids, carbohydrates, sterols/terpenes, and tannins. Whereas, alkaloids, amino acids and resins were not detected. Present components were quantified and its dry weight, related to 1 g of aqueous extract lyophilized results as follow: flavonoids 76.5 ± 3.2 mg/g, carbohydrates 4.3 ± 0.7 mg/g, sterols/terpenes 244.4 ± 16.0 mg/g, and tannins 27.1 ± 1.3 mg/g.
We next performed the standard MTT assay to demonstrate aqueous extract cytotoxicity against human cell lines (Fig. 1). A comparative study between treatments with T. absinthioides and 5-FU was completed on tumoral cells HeLa, Gli-36, HCT-116 and MCF-7; and non-tumoral
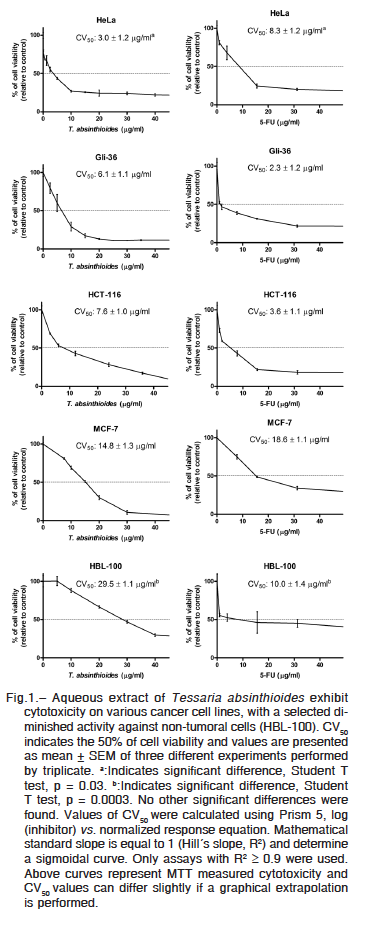
HBL-100 cells. Induced cytotoxicity by T. absinthioides extract was strong in Hela and Gli-36 cells (CV50: 3.0 ± 1.2 and 6.1 ± 1.1 μg/ml, respectively), intermediate in HCT-116 and MCF-7 (7.6 ± 1.0 and 14.8 ± 1.3 μg/ml, respectively) and moderate on HBL-100 (29.5 ± 1.1 μg/ml). Meanwhile, a different pattern of response was observed when 5-FU was a treatment. The CV50 calculated by cell line, was: Gli-36, 2.3 ± 1.2 μg/ml; HCT-116, 3.6 ± 1.1 μg/ml; HeLa, 8.3 ± 1.2 μg/ml; HBL-100, 10.0 ± 1.4 μg/ml; and MCF-7, 18.6 ± 1.1 μg/ml.
The present data demonstrate that uterine cervix carcinoma and breast cancer cell lines were more sensitive to T. absinthioides than to 5-FU. In Hela cells the difference was significant (Student T Test, p = 0.03). On the contrary, in glioblastoma, colorectal cancer and non-tumoral cell lines 5-FU results more cytotoxic than the plant extract. Notably, when a normal cell line HBL-100 was tested, the measured CV50 induced by T. absinthiodes resulted significantly higher (3 folds) than the 5-FU obtained value (Student T Test, p = 0.0003). An increase in CV50 indicates lower cytotoxicity, when the effect is observed in non-tumoral cells (HBL-100), these results indicate a T. absinthiodes selective toxicity against cancer cells.
Then, we performed neurobehavioral, anatomical, hematological and serological studies on Sprague Dawley healthy rats to examine possible alterations induced by T. absinthioides treatment, with special attention in toxic signs similar to those reported for 5-FU treatment.
One dose by gavage of aqueous extract, at concentrations of 0, 50, 300, or 2000 mg/kg/bw did not determine acute toxicity. No females or males died as consequence of extract administration or into the period of 14 days after treatment; no animals showed neurobehavioral signs of toxicity. After necropsy, internal organs were analyzed and no-macroscopical signs of ischemia, bleeding, fibrosis or degenerative proliferation were observed. In consequence, it is possible to discard acute toxicity induced by oral administration of T. absinthioides up to 2000 mg/kg/bw.
Thereafter, we perform a dose-repeated-28-days study of toxicity. During the assay period, neuro-behavioral signs of toxicity were not registered and sensory/motor functions did not show alterations. When treatment finished, anatomo-pathological, blood and serological studies were performed to analyze whether treatment induce toxicity in detoxifying organs, such as liver, kidneys and lungs, or into a rapidly proliferating fraction of cells, such as spleen and circulating blood cells. Glucose, hepatic enzymes, urea and creatinine were determined to analyze liver and kidneys functional status. Serum values of glucose and urea were determined to evaluate metabolism integrity (Table 1). No toxic changes were registered at any used doses. Analytical results obtained at 0 and
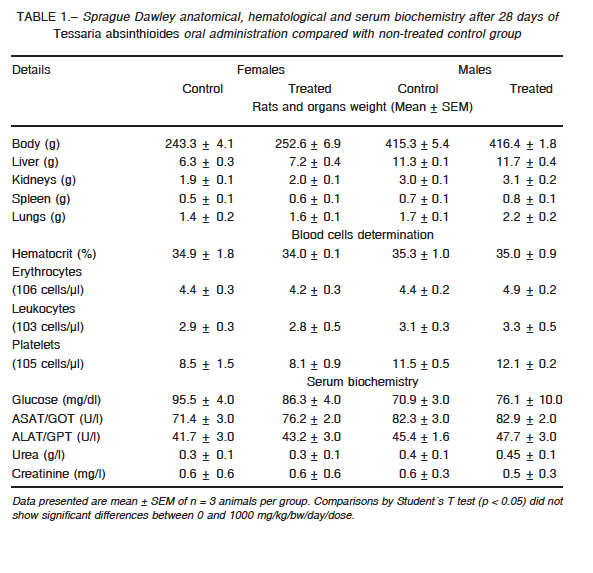
1000 mg/kg bw/day are showed in Table 1. Final weight of females and males did not show significant changes. The organs of treated animals did not evidence differences in weight, color or structure. No macroscopical signs of ischemia, bleeding, fibrosis or degenerative proliferation were observed.
Hematocrit and the number of circulating blood cells were similar after treatment. Extract toxicity on rapidly proliferating cells or organs related to the hematological system can therefore be excluded. None of the assessed biochemical parameters showed statistical differences between groups.
All the parameters had no significant differences when 0 and 1000 mg/kg dose/bw/day were compared (Student´s T test, p ≤ 0.05). Moreover, determined parameters are considered normal to the specie24.
Concluding, this section demonstrates that oral administration of T. absinthioides aqueous extract, for 28 days, it is not toxic at doses up to 1000 mg/kg bw/day.
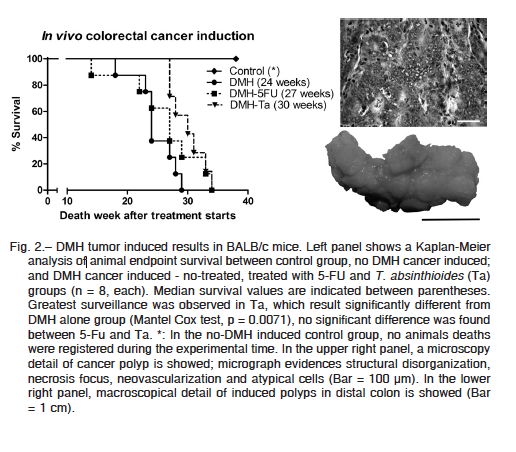
After acute and 28 repeated-dose toxicity was discarded, to evaluate the capability of aqueous extract to exert in vivo antitumoral activity, we induced colorectal tumors in BALB/c mice by DMH administration. In the control group (animals without DMH tumoral induction) animals presented 100% survival. No spontaneous animal deaths were registered, and no animals evidence the humane endpoints criteria during the experimental time (52 weeks after tumor induction start).
All animals treated with DMH developed colorectal cancer and were sacrificed as consequence of tumor disease. Necropsy of animals evidenced 10 to 24 colorectal polyps (Fig. 2, right lower panel), without significant differences between groups; neither difference in polyps´ volume were found. Histologic analysis of tumoral pieces confirms the diagnosis of colorectal adenocarcinoma (Fig. 2, right upper panel).
Only the median survival was different between the groups in which tumors were induced (Fig. 2, left panel). While no treated group median survival was 24 weeks after treatment starts, in 5-FU and T. absinthioides groups were 27 and 30, respectively. Differences between no treated group and T. absinthioides were significant (Mantel Cox test, p = 0.0071). The presented data clearly indicate the antitumoral effects of T. absinthioides when is orally administrated. When T. absinthioides group is compared with 5-FU group, no significant differences were found (Mantel Cox test, p = 0.1179).
Discussion
In accordance with our results, T. absinthioides aqueous extract has a selective cytotoxicity against different human tumoral cell lines showing a reduced activity in non-tumoral cells. In addition, oral administration of aqueous extract does not determine acute or subchronic toxic effects in laboratory healthy animals; whereas, the extract demonstrates in vivo antitumoral activity by increasing the median survival of BALB/c mice with colorectal cancer.
Both, cytotoxic in vitro and antitumoral in vivo results, were compared with the worldwide distributed chemotherapeutic, 5-FU. The antimetabolite 5-FU acts by inhibition of thymidylate synthase enzyme, thus preventing DNA synthesis25. It is widely used in the treatment of various
malignancies like gastrointestinal, breast, colorectal, liver, skin, head and neck cancers26. Although 5-FU is generally well tolerated in vivo, it also possesses severe side effects such as: nephrotoxicity, hepatotoxicity, ototoxicity, and neurotoxicity27. T. absinthioides demonstrated activity, allowed us to compare its efficiency against cancer cells with 5-FU, with a reduced or absent secondary undesirably effects against non tumoral and healthy tissues.
Because T. absinthioides evidenced anticancer activity, a new window of cancer research was opened for this plant. It is remarkable that 61% of anticancer agents approved are natural derived products28. Some phytochemical components of the T. absinthioides aqueous extract are widely reported as active compounds against cancer cells. As examples, flavonoids actions are related to direct free radical scavenger, cytotoxicity and protection against pro-carcinogenic compounds29, 30; terpenes and sterols were described as cytotoxic compounds against several tumoral cells and potentiating pro-apoptotic agents31-33; while, vegetal derived tannins were demonstrated to act as cytotoxic by induction of antiproliferative and pro-apoptotic actions34, 35. In accordance to the exposed antecedents, reported chemical groups derived from T. absinthioides aqueous extract, represent a promissory field of study for plant-derived anti-cancer products.
Although, some in vitro studies were published using T. absinthioides natural derivatives, there were not previous reports of significant cytotoxicity against tumoral cell lines. Describing virucidal activity, García et al.9, 10 reported lack cytotoxicity for Vero cells of the essential oils extracted from T. absinthioides. Other studies demonstrate the antiproliferative activity of semi-synthetic molecules derived from tessaric acid obtained from aerial parts of the plant; sensitive cell lines were A2780 (ovarian cancer), HBL-100 (non-tumoral epithelium), HeLa (uterine cervix adenocarcinoma), SW1573 (lung alveolar carcinoma), T-47D (breast adenocarcinoma) and WiDr (colorectal adenocarcinoma); curiously, in the same work, the natural derivative tessaric acid was reported as inactive36. Other study performed by Torres Carro et al.8 reported nontoxic activity of hydro-alcoholic extract of T. absinthioides on RAW 264.7 (murine macrophages) at doses up to 200 μg/ml; interestingly, phytochemical identification of this extract reported flavonoids, tannins, but not sterol/terpenes contents. In consequence, the cytotoxic results presented in the current work represent a valuable evidence of T. absinthioides natural extract activity on cancer cells. In relation to the cytotoxicity, it is important to remark, the capability of the aqueous extract to exert a selective cytotoxicity on tumoral cells. While the 5-FU shows an intermediate cytotoxicity on HBL-100 cell line, similar to the measured for HeLa uterine cervix cancer cells, the CV50 of T. absinthioides extract was the highest on non tumoral cells, been the double than values obtained for the extract on MCF-7 cells; and the triple of the value presented by 5-FU on the HBL-100. Altogether, the evidence show in this work make the aqueous extract an important material to perform bio-guided fractionation procedures destined to isolate and identify specific chemical molecules with oncologic relevance.
On the other side, the present results show that acute and repeated-doses of T. absinthioides do not exert undesirable effects; while it is widely accepted that, in laboratory animals, the common clinical side effects of 5-FU include myelosuppression, diarrhea, vomiting, mucositis, cardiotoxicity and neurotoxicity37. The obtained data about non-toxic consumption of T. absinthioides support their ethnopharmacological, culinary and biomedical uses. Moreover, they validate the in vivo experimentation of previous reported antiviral, anti-inflammatory and gastric cytoprotective agent properties7, 8, 11, 38. About its use as insecticidal9, 10, the results shown herein provide experimental data that exclude induction of toxic effects on vertebrate animals. Overall, the safety oral administration, analyzed together with the cytotoxicity against cancer cells and extract antitumoral effect, constitute strong evidence to promote investigation of T. absinthioides plant derivatives and its biomedical and biological properties.
When the antitumoral effects are considered, the median survival increase obtained by oral administration of T. absinthioides, indirectly indicates bioavailability, stability and activity of natural plant derivative/s on in vivo models. In the context of cancer research, the in vivo confirmation of the hypothesized antitumoral properties represents a critical result to ensure compound efficacy.
Despite its in vivo results, and the cytotoxic effects described against the human colorectal cancer cell line (HCT-116), it is not possible to stablish a clear correlation between the effective doses used in both models. In fact, the efficiency of in vitro cytotoxic assays to predict animal toxicity remains controversial39-42. In accordance with Huntjens et al.43, we consider that pharmacokinetic and pharmacodynamic data form the basis for scaling and predicting drug effects in vivo. Although, much more information needs to be explored about T. absinthioides antitumoral actions, the present work represents a well demonstrated report of plant value into the cancer research.
Altogether, the results presented in the current work open a new window about this plant research into the oncology field. Deep studies are required to elucidate the chemical structure of bioactive molecules, its pharmacological properties, the specific cellular responses, its related molecular pathways, and the capability of the compound to potentiate others current used chemotherapeutic agents.
In conclusion, the present work is a relevant report of T. absinthioides aqueous extract activity related to cancer. The natural compound evidence cytotoxic activity against different human tumoral cell lines, representative of glioblastoma, uterine cervix, colorectal and mammary adenocarcinomas; with a selective and significant diminished effects on non tumoral cells. Acute and dose-repeated in vivo administration of aqueous extract it is not toxic; in healthy animals, administration of plant derivatives does not induce neurobehavioral, anatomical, hematological, serological nor lethal effects. In mice induced colorectal cancer, oral administration of extract increases significantly the median survival. Altogether, the current work make the T. absinthioides derived products promising for cancer research and treatment.
Acknowledgements: This work was supported by 06/J473 grant, from Secretaría de Ciencia, Técnica y Postgrado (SeCTyP) – Universidad Nacional of Cuyo – Argentina; PICT2014-1877 grant, from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and Instituto Nacional del Cáncer (INC) grant, both from Ministerio de Ciencia, Tecnología e Innovación Productiva – Argentina; and grant 11220150100579CO, from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Conflict of interests: None to declare.
References
1. Kinghorn AD, Pan L, Fletcher JN, et al. The relevance of higher plants in lead compound discovery programs. J Nat Prod 2011; 74: 1539-55.
2. Kurina Sanz MB, Donadel OJ, Rossomando PC, et al. Sesquiterpenes from Tessaria absinthioides. Phytochemistry 1997; 44: 897-900.
3. Bailac P, Duschatzky C, Carrascull A, et al. Composition of the Essential Oils of Tessaria absinthioides (Hook et Arn.) D. Candole. J Essent Oil Res 2013; 10:89-91.
4. Barboza GE, Cantero JJ, Núñez C, et al. Medicinal plants: a general review and a phytochemical and ethnopharmacological screening of the native argentine flora. Kurtziana 2009; 34: 365.
5. Campos-Navarro R, Scarpa GF. The cultural-bound disease “empacho” in Argentina. A comprehensive botanico-historical and ethnopharmacological review. J Ethnopharmacol 2013; 148: 349-60.
6. Madaleno IM, Delatorre-Herrera J. Popular medicine of Iquique, Tarapacá. Idesia 2013; 31: 67-78.
7. Visintini Jaime MF, Redko F, Muschietti LV, et al. In vitro antiviral activity of plant extracts from Asteraceae medicinal plants. Virol J 2013; 27: 245.
8. Torres Carro R, Isla MI, Ríos JL, et al. Anti-inflammatory properties of hydroalcoholic extracts of Argentine Puna plants. Food Res Int 2015; 67: 230-7.
9. García M, Sosa ME, Donadel OJ, et al. Effects of some sesquiterpenes on the stored-product insect Tenebrio molitor (Coleoptera: Tenebrionidae). Rev Soc Entomol Argent 2003; 62: 17-26.
10. García M, Sosa ME, Donadel OJ, et al. Allelochemical effects of eudesmane and eremophilane sesquiterpenes on Tribolium castaneum larvae. J Chemical Ecology 2003; 29: 175-87.
11. Donadel OJ, Guerreiro E, María AO, et al. Gastric cytoprotective activity of ilicic aldehyde: structure–activity relationships. Bioorg Med Chem Lett 2005; 15: 3547-50.
12. Afaq SH, Siddiqui MMH, Tajuddin SA. In: Publication Division ed, Standardization of Herbal Drugs.1st ed. Aligarth: AMU Press, 1994, p 66,100,143-146.
13. Evans WC. Alkaloids. In: Saunders W.S, ed. Trease and Evans Pharmacognosy, 15th ed. New York: Elsevier Health Science, 2002, pp 333-93.
14. Fornasworth WR. Biological and phytochemical screening of plants. J Pharma Sci 1966; 55: 225.
15. Khan R, Zakir M, Afaq SH, et al. Activity of solvent extracts of Prosopis spicigera, Zingiber officinale and Trachyspermum ammi against multidrug resistant bacterial and fungal strains. J Infect Dev Ctries 2010; 4: 292-300.
16. Herborne JB. The terpenoids. In: Jeffrey Barry, ed. Phytochemical methods: A Guide to Modern Techniques of Plant Analysis, 1st ed. London-New York: Chapman and Hall Ltd, 1973, pp 90-131.
17. Evans WC. Carbohydrates. In: Saunders W.S, ed, Trease and Evans Pharmacognosy. 15th ed. New York: Elsevier Health Science, 2002, p 191-213.
18. Evans WC. Basic metabolic pathways and the origin of secondary metabolites. In: Saunders W.S, ed. Trease and Evans Pharmacognosy. 15th ed. New York: Elsevier Health Science, 2002, pp 150-69.
19. Evans WC. Saponins, cardioactive drugs and other steroids. In: Saunders W.S, ed. Trease and Evans Pharmacognosy. 15th ed. New York: Elsevier Health Science, 2002, p 289-314.
20. Tambe VD, Bhambar RS. Estimation of total phenol, tannin, alkaloid and flavonoid in Hibiscus Tiliaceus Linn. Wood extracts. Journal of Pharmacognosy and Phytochemistry 2014; 2: 41-7.
21. OECD (Organization for Economic Cooperation and Development). Guideline for the Testing of Chemicals. Test N° 420: Acute Oral Toxicity – Fixed Dose Procedure. 2002. In: http://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788; consulted 29/03/2017.
22. OECD (Organization for Economic Cooperation and Development). Guideline for the Testing of Chemicals. Test N° 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents. 2008. In: http://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788; consulted 29/03/2017.
23. Deng S, Hu B, An HM, et al. Teng-Long-Bu-Zhong-Tang, a Chinese herbal formula, enhances anticancer effects of 5-Fluorouracil in CT26 colon carcinoma. BMC Complement Altern Med 2013; 13: 128.
24. Sharp PE, La Regina MC. In: Laboratory rat. Boca Raton (US): CRC Press LLC, 1998.
25. Lemaire L, Malet Martino MC, Forni MD, et al. Cardiotoxicity of commercial 5-fluorouracil vials stems from the alkaline hydrolysis of this drug. Br J Cancer 2000; 66: 119-27.
26. Miura K, Kinouchi M, Ishida K, et al. 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers (Basel) 2010; 2: 1717-30.
27. Wang S, Zhu F, Marcone MF. Staghorn sumac reduces 5-fluorouracil-induced toxicity in normal cells. J Med Food 2015; 18: 938-40.
28. Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res 2006; 20: 1023-35.
29. Alhusainy W, Williams GM, Jeffrey AM, et al. The natural basil flavonoid nevadensin protects against a methyleugenol-induced marker of hepatocarcinogenicity in male F344 rat. Food Chem Toxicol 2014; 74: 28-34.
30. Kaur P, Kaur V, Kumar M, et al. Suppression of SOS response in E. coli PQ 37, antioxidant potential and antiproliferative action of methanolic extract of Pteris vittata L. on human MCF-7 breast cancer cells. Food Chem Toxicol 2014; 74: 326-33.
31. Pacifico S, Gallicchio M, Lorenz P, et al. Apolar Laurus nobilis leaf extracts induce cytotoxicity and apoptosis towards three nervous system cell lines. Food Chem Toxicol 2013; 62: 628-37.
32. Geromichalos GD, Papadopoulos T, Sahpazidou D, et al. Crocus sativus L constituent suppresses the growth of K-562 cells of chronic myelogenous leukemia. In silico and in vitro study. Food Chem Toxicol 2014; 74: 45-50.
33. Do MT, Na M, Kim HG, et al. Ilimaquinone induces death receptor expression and sensitizes human colon cancer cells to TRAIL-induced apoptosis through activation of ROS-ERK/p38 MAPK-CHOP signaling pathways. Food Chem Toxicol 2014; 71: 51-9.
34. Barrajón-Catalán E, Fernández-Arroyo S, Saura D, et al. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem Toxicol 2010; 48: 2273-82.
35. González-Sarrías A, Yuan T, Seeram NP. Cytotoxicity and structure activity relationship studies of maplexins A-I, gallotannins from red maple (Acer rubrum). Food Chem Toxicol 2012; 50: 1369-76.
36. León LG, Donadel OJ, Tonn CE, et al. Tessaric acid derivatives induce G2/M cell cycle arrest in human solid tumor cell lines. Bioorg Med Chem 2009; 17: 6251-6.
37. Al-Hamdany MZ, Al-Hubaity AY. The structural changes of the rat’s lung induced by intraperitoneal injection of 5-fluorouracil. J Pak Med Assoc 2014; 64: 734-8.
38. García CC, Talarico L, Almeida N, et al. Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother Res 2003; 17: 1073-5.
39. Phillips JC, Gibson WB, Yam J, et al. Survey of the QSAR and in vitro approaches for developing non-animal methods to supersede the in vivo LD50 test. Food Chem Toxicol. 1990; 28: 375-94.
40. Barile FA, Arjun S, Hopkinson D. In vitro cytotoxicity testing: biological and statistical significance. Toxic in Vitro 1993; 7: 111-16.
41. Garle MJ, Fentem JH, Fry JR. In vitro cytotoxicity tests for the prediction of acute toxicity in vivo. Toxic in Vitro 1994; 8: 1303-12.
42. Spielmann H, Genschow E, Liebsch M, et al. Determination of the starting dose for acute oral toxicity (LD50) testing in the up and down Procedure (UDP) from cytotoxicity data. ATLA 1999; 27: 957-66.
43. Huntjens DRH, Spalding DJM, Danhof M, et al. Correlation between in vitro and in vivo concentration-effect relationships of naproxen in rats and healthy volunteers. Brit J Pharm 2006; 148: 396-404.
– – – –
We hold that one type of intelligent occupation should, in all but exceptional cases, increase the capacity for comprehension in general; that it is an error to segregate minds of men into rigid guild classifications; and that art and sciences have much in common and both may profit by mutual appraisal. The Europeans have long appreciated this. That our book has contributed in this respect we have not the temerity to assert. At any rate, we have written along as it suited our fancy, and have been amused and rested in so doing.
Sostenemos que un tipo de ocupación inteligente, a no ser en casos excepcionales, aumenta la capacidad de comprensión en general, y que es un error segregar las mentes de los hombres en rígidas clasificaciones por gremios y que el arte y las ciencias tienen mucho en común y pueden ambas beneficiarse por mutuo acercamiento. Los europeos hace rato que apreciaron esto. Que nuestro libro haya contribuido en este respecto no tenemos la temeridad de afirmarlo. De todas maneras, lo hemos escrito a nuestro gusto y nos hemos entretenido y descansado haciéndolo.
Hans Zinsser (1878-1940)
Rats, Lice and History (1935): Being a Study in Biography, which, after Twelve Preliminary Chapters Indispensable for the Preparation of the Lay Reader, Deals With the Life History of TYPHUS FEVER. New York: Black Dock & Leventhal, 1996. Preface, p IX
