VERÓNICA FLORES1, 4, GUSTAVO VIOZZI1, 4, GILDA GARIBOTTI2, DANIELA ZACHARIAS2,
MARÍA FLORENCIA DEBIAGGI3, 4, SURPIK KABARADJIAN5
1Laboratorio de Parasitología, INIBIOMA (CONICET-UNCo), 2Departamento de Estadística, Universidad Nacional del Comahue, 3Cátedra de Microbiología y Parasitología, Facultad de Ciencias Médicas, Universidad Nacional del Comahue, 4Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), 5Unidad Regional de
Sanidad Ambiental de la Provincia de Río Negro, Río Negro, Argentina
Abstract In urban populations of South America, dogs with free access to public areas represent a public
health concern. The primary consequence of roaming dogs on human health is the transmission of infectious and parasitic diseases mainly through feces contamination. The main diseases likely to be transmitted are hydatidosis or echinococcosis, larva migrans, and giardiasis. In Argentina, hydatidosis ranks among the most prevalent zoonosis. Although it is considered a rural disease, the circulation of this parasite in urban areas has been documented. The aim of this work was to survey intestinal parasites in canine feces from two low-income urban neighborhoods of Bariloche city, Argentina, and to assess their seasonal variation. During 2016, 188 fresh dog feces were collected from sidewalks in 40 randomly selected blocks from the neighborhoods. Each sample was processed by Sheater flotation and tested for a coproantigen (CAg) by ELISA. The percentage of parasitized feces was 65.3% (95% CI: 55.9%-73.8%). Eleven parasite species were found, 3 protozoan, 3 cestodes, and 5 nematodes. Echinococcus sp. was present in 9.3% of the samples (95% CI: 4.7%-16.1%). Canine echinococcosis rates resulted similar to rates found previously in other neighborhoods of the city. The life cycle of Echinococcus sp. is sustained in urban areas by the entry of parasitized livestock, domiciliary slaughtering, and inadequate deposition of offal. The risk of Echinococcus sp. transmission to people in these neighborhoods is very high, due to high density of free-roaming dogs and high percentages of infected feces, similar to percentages observed in rural areas.
Key words: zoonosis, stray dogs, dog feces, Echinococcus sp., low-income populations
Resumen Echinococcosis y otros parásitos que infectan a perros domésticos en áreas urbanas de
una ciudad de la Patagonia argentina. En las poblaciones urbanas de América del Sur, los perros con acceso libre a áreas públicas representan un problema de salud pública. La principal consecuencia es la transmisión de enfermedades infecciosas y parasitarias a través de la contaminación por heces. Las principales enfermedades que pueden transmitirse son hidatidosis, larva migrans y giardiasis. En Argentina, la hidatidosis es una de las zoonosis más prevalentes y aunque es considerada una enfermedad rural, algunos estudios muestran la circulación de este parásito en zonas urbanas. El objetivo fue registrar los parásitos intestinales en heces caninas de dos barrios de bajos ingresos de la ciudad de Bariloche, Argentina, y evaluar su variación estacional. Durante 2016, se recolectaron 188 heces frescas de perros en 40 manzanas seleccionados aleatoriamente. Las heces se procesaron mediante flotación de Sheater y una prueba ELISA de coproantigeno (CAg). El porcentaje de heces parasitadas fue del 65.3% (IC 95%: 55.9%-73.8%). Se encontraron 11 especies de parásitos, 3 protozoos, 3 cestodes y 5 nematodes. Echinococcus sp. estuvo presente en el 9.3% de las heces (IC 95%: 4.7% -16.1%). La equinococosis canina mostró valores similares a estudios previos en otros barrios de la ciudad. El ciclo de vida Echinococcus sp. se mantiene en las zonas urbanas por entrada de ganado parasitado, faena domiciliaria y deposición inadecuada de vísceras. El riesgo de transmisión de Echinococcus sp. en estos barrios es alto, debido a la alta densidad de perros sueltos y al alto porcentaje de heces infectadas, similar al de las zonas rurales.
Palabras clave: zoonosis, perros sueltos, heces caninas, Echinococcus sp., poblaciones de bajos ingresos
Received: 4-VIII-2017 Accepted: 17-X-2017
Postal adress: Verónica Flores, Laboratorio de Parasitología, INIBIOMA (CONICET-UNCo), Quintral 1250, 8400 Bariloche, Río Negro, Argentina
e-mail: veronicaroxanaflores@gmail.com
In urban and suburban populations of South America, companion animals and dogs with free access to public areas pose a public health risk. The main consequences of roaming dogs on human health are the transmission of infectious and parasitic diseases, the generation of unhealthy foci due to open trash bags, bites, and traffic accidents1.
Parasitic diseases are an important cause of morbidity. Poverty, poor personal hygiene, lack of sanitation and potable water supply, productive activity and habits of the community, along with feeding and defecation practices of pets play a significant role in the transmission of zoonotic diseases2. The main diseases likely to be transmitted by dogs are hydatidosis, larva migrans (toxocariasis and ancylostomiasis), and giardiasis2-5. In Patagonian cities, the zoonotic protozoa and helminthes recorded to date in canine feces are: Sarcocystis spp., Entamoeba spp., Giardia spp., Isospora spp., Toxocara canis, Toxascaris leonina, Trichuris vulpis, Capillaria spp., Uncinaria sp., Ancylostoma caninum, Strongyloides sp., Dypillidium caninum, Taenia spp., Diphyllobothrium sp., and Echinococcus sp.6-10.
Hydatidosis is a zoonotic disease caused by species of the genus Echinococcus. The human infection occurs after the ingestion of Echinococcus eggs through contaminated foods or by direct contact with parasitized dogs. Hydatidosis produces morbidity, disability and death, affecting the health of the population and the regional economy3, 11. Echinococcosis is a cosmopolitan zoonotic disease and one of the most prevalent zoonosis in rural areas from southern Brazil, Chile, Peru, Uruguay, and Argentina3. Risk and prevention factors have been extensively studied in rural areas of these countries. The main risk factor is age of children, while drinking water at home appears as a protection factor3. The most effective and efficient way to control hydatidosis is primary prevention. Thus, it is indispensable to develop health education to motivate the population to cooperate in avoiding infection of children, dogs, and peridomicile3. Although hydatidosis is considered a rural disease12, some studies show the circulation of the parasite in urban areas10. In Europe, Echinococcus multilocularis reached urban areas with the invasion of foxes and dogs preying on infected rodents12. In developing countries, the habit of slaughtering livestock at home in urban areas, and feeding dogs with infected viscera, could favor the translocation of E. granulosus to cities13-16. Caldas et al.11 reported that between 5.7% and 19.3% of dogs from different rural areas in Argentina were infected with E. granulosus. In this country, many deaths occur each year from hydatidosis, with a death rate of 2.7% between 2009 and 201311. Studies in Patagonia found that between 1.2% and 11.0% of dog feces in urban areas are infected with echinococcosis10, 17.
In the Province of Río Negro, Argentina, the hydatidosis control program was launched in 1980 primarily aimed to deworm dogs. In 1986, ultrasound surveys of children showed a prevalence of 5.6%, and the percentage dropped to 0.3% after a decade of government control programs13, 18, 19.
Low income neighborhoods of Bariloche city, Río Negro, are characterized by rural migration and flow between the countryside and the city. People in these neighborhoods preserve many rural practices such as domiciliary slaughter of sheep and goats20. A parasitological study of dog feces in a low-income neighborhood of Bariloche found that 11.0% were infected with E. granulosus10. A demographic survey of canine population performed in two low income neighborhoods showed that 87% of the houses have at least one dog, with a household/dog ratio of 2.2, and 56% of owned dogs are allowed to roam in the streets21. Therefore, it is an ideal scenario for transmission of zoonotic parasites. The objective of this study is to continue a survey of intestinal parasites in dog feces in neighborhoods from Bariloche. In this opportunity, we focus on two low-income neighborhoods assessing seasonal changes in parasite prevalence. Special emphasis is put on the presence of echinococcosis for its consequences on public health, including the discussion of risk factors for humans and measures to avoid human infection.
Materials and methods
The selected neighborhoods are located in the southern side of Bariloche city (41° 10’S – 71° 18’W) (Fig. 1). They comprise an area of 85 blocks (100 m x 100 m each). In 2010 the population of the study area was of 5877 people22. These are fast growing neighborhoods with an estimated 20% population increase in the last 6 years. We assume that about 7100 people inhabit the area nowadays. According to the 2010 census22 between 15% and 57% of the households had at least one unsatisfied basic need.
Forty blocks in the study area were randomly selected. Canine feces were collected in summer, fall, and winter, 2016. One fresh feces sample was gathered from the sidewalk of each block in each of the three seasons. A total of 118 samples were obtained: summer (N = 38), fall (N = 40), and winter (N = 40).
Each feces sample was collected in an individual bag and kept frozen until processed. The Sheater flotation method was used. Two slides of each sample were microscopically examined at 100X and 400X amplifications. Identification of eggs, cysts, and larvae of parasites was performed by morphological characteristics23.
In order to determine the presence of Echinococccus sp., each fecal sample was mixed in equal parts of 0.15 M PBS with 0.3% Tween 20, vortexed vigorously, and centrifuged at 3500 rpm for 30 minutes at room temperature. The supernatant was removed and stored at -20 °C until further processed. The Coproantigen (CAg) ELISA test was performed according to Pierangeli et al24. All fecal samples and controls were analyzed in duplicate. Samples with an optical density (OD) value above or equal to the optimal cut off value (OD 0.235) were classified as positive.
Exact binomial 95% confidence intervals (95% CI) were calculated for the overall percentage of infected feces and by parasite species. Prevalence of parasitized feces between studied periods were compared using the Exact Fisher test. All analyses were performed with the R 3.2.2 package25.
Results
Eleven parasite species were recorded: 3 protozoan, 3 cestodes, and 5 nematodes. A total of 77 parasitized feces were found (65.3%, 95% CI = 55.9%-73.8%). Single infections were present in 42 feces (35.6%, 95%CI:
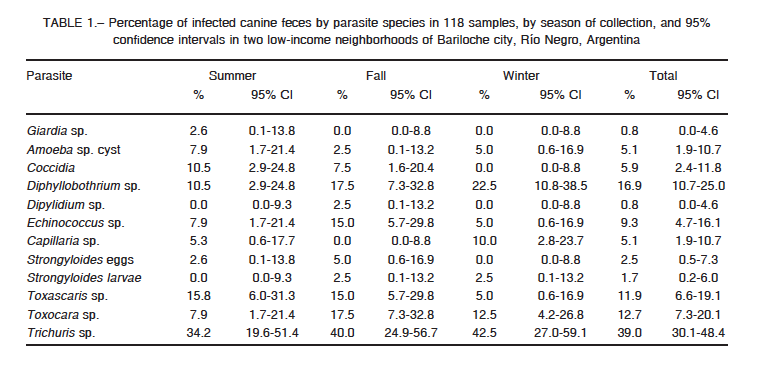
27.0%-44.9%) while multiple infections were observed in 35 feces (29.7%, 95% CI: 21.6%-38.8%). For each parasite species, the percentage and 95% confidence interval of infected feces overall and by month of survey, are presented in Table 1. The most frequent parasite was Trichuris sp. (39%) followed by Diphyllobothrium sp. (17%). The percentage of feces infected with Echinococcus sp. was 9.3%.
The overall percentage of infected samples in summer was 60.5% (95% CI: 43.4%-76.0%), fall 70.0% (95% CI: 53.5%-83.4%) and winter 65.0% (CI = 48.3%-79.4%). The variation was not statistically significant (p = 0.6963). The per species analysis of season variation was not statistically significant for any of them (p-value > 0.1 for all species). Giardia sp. was found only during the summer, while Dipylidium caninum was found only in the fall. It is worth noting that the most zoonotic species (Amoeba sp. cysts, Echinococcus sp., and Toxocara sp.) were found in the three studied seasons.
Discussion
Studies performed in cities from Southern Argentina and Southern Chile found that the average numbers of dogs per household range between 0.6 and 1.4, rising up to 2.4 in some neighborhoods26-28. Although this is a problem per se, the main problem is related to the characteristics of pet ownership. The percentage of owned dogs allowed to roam freely found in these studies varies between 22% and 51%26-28. There are no estimates of demographic parameters of the dog population in Bariloche. However, visual inspection and surveys performed in some neighborhoods give a clear indication of a street dog problem21.
The richness in parasite species (11) in our study was higher than observed in Mexico29 (6 species) and Peru30 (5 species) but lower than species richness reported in Santiago, Chile where 16 different parasite species were found in dog feces31. However, it is worth noting that this latter work analyzed 972 dogs with gastrointestinal symptoms. The richness of parasite species in dog feces from Patagonian cities varies between 10 and 117, 9, 32. The estimated overall percentage of infected feces in the neighborhoods studied herein was 65.3%, a value which is within the range of other cities in the Patagonian region; for example, the prevalence of infected feces reported in Comodoro Rivadavia, Chubut, was found to range between 46.6% and 86%7, 32. This last value was obtained in a very low-income neighborhood near the urban solid waste disposal facility. The percentages of infected feces were also similar to those found in other Latin American studies like Mexico (73.3%), Peru (40.1%), and Chile (64.8%)29-31. Previous studies in other neighborhoods of Bariloche found between 37% and 67% of parasitized feces, and the composition of the parasite communities was very similar to the one observed in this study10.
As in previous works, no clear seasonal infection patterns were observed in our study, as seasonal variations did not reach statistical significance in parasites recovered throughout the study period. Similar results were reported in other countries with temperate climate like the USA and Spain. Seasonal variations were reported for ascarids and Giardia sp. with higher infection values in fall and winter33, 34. We found the ascarid Toxocara sp. in all three seasons with the highest infection value in fall. In contrast, we only recorded the presence of Giardia sp. in summer. Hookworms also show different seasonal infection patterns around the world; e.g. in the USA these species showed maximum infection values in spring and summer33 while in Spain A. caninum showed maximum values in fall and winter34.
The World Health Organization (WHO) suggested that the control of echinococcosis is a priority within the framework of “care for neglected diseases in deferred populations”11. As mentioned, echinococcosis is considered a rural zoonosis; however, numerous cases were found among both urban dogs and people who never left the cities, indicating that the disease is cycling in urban areas of South America35, 36. In Peru, stray dogs infected with E. granulosus were found in areas surrounding slaughterhouse, where offal of infected animals is improperly discarded due to non stringent supervision; moreover it is possible that infected offal reaches city markets through informal channels36, 37. The percentage of dog feces infected with Echinococcus sp. in our study was 9.3%, similar to values reported in urban areas of Chile (8-15%)15, but higher compared to Bolivia (3.4%) and Brazil where no CAg was found in feces16, 38. The prevalence of echinococcosis in our study is 10 times higher than values reported in other cities of the region9, 16, 17, 32, and similar to prevalences in rural areas9, 16, 32, 39-40. A previous study in other low-income neighborhoods of Bariloche found a similar prevalence of echinococcosis in dog feces10, showing persistent transmission foci in urban neighborhoods with rural habits due to human mobility between the countryside and the city20.
In Argentina, human cases of hydatidosis have been detected in cities16, particularly in children under 10-year-old, and many of these cases were unrelated to rural areas. This situation is relatively recent, thus Echinococcus sp. life cycle is maintained in urban areas subsidized by uncontrolled entry of parasitized livestock, domiciliary slaughtering, inadequate deposition of offal16, and by the lack of knowledge on the risk involved in these practices, along with inadequate dog care. In the neighborhoods included in this study, people who never left the city were found to harbor cysts (personal observation).
These neighborhoods are characterized by an expansive population pyramid (almost 30% under 12-year-old) and low educational level. These demographic characteristics are factors favoring the risk of transmission of zoonotic diseases, especially hydatidosis3. Many of the preventive measures are difficult to apply. For example, frequent washing of hands and vegetables is not possible in some households because 22.1% lacks home water supply and 11.4% lacks access to safe drinking water41. Regarding preventive measures related to dog care, almost half of the dog population has free access to the street21. Although 83.4% of the dogs were dewormed, they were treated only once during the previous year21, while the recommendation is to deworm free roaming dogs at least 4 times a year42. Besides, there is no government deworming control program for dogs in the city. Also, sheep slaughter and fish evisceration with inadequate offal and discard disposal are rather common practices in these neighborhoods. In agreement with these observations, we found Diphyllobothrium sp. in 16.9% of the feces, an infection acquired by dogs consuming raw viscera of infected fish, and 9.3% of feces were positive for Echinococcus sp. Thus, the dispersion of parasite eggs is promoted by large numbers of dogs roaming without control21; it should be taken into account that dogs defecate within 200 m of their houses43. Additionally, the climate in Bariloche favors parasite egg survival; indeed, eggs were shown to survive long in the environment at low temperatures (7 °C) and remain viable for up to 294 days3. More recently, some studies have found that eggs can remain infective after 41 months under arid conditions in certain regions of the Argentine Patagonia3.
In conclusion, in this study we found high rates of parasite infected dog feces, including potentially zoonotic species, in two low-income neighborhoods of Bariloche. These findings raise a public health alert. Several factor –namely high numbers of dogs roaming freely in the streets, lack of systematic canine deworming, domiciliary livestock slaughter and inadequate disposal of offal, among others– provide suitable conditions for the maintenance of parasites in the urban environment. Joint governmental-private initiatives should be promoted in order to address this multifactorial problem in low-income neighborhoods of Bariloche.
Acknowledgements: This work was supported by Dirección Nacional de Desarrollo Universitario y Voluntariado [V8-UNCOMA749]; and by Universidad Nacional del Comahue [UNCo B 187].
Conflicts of interest: None to declare
References
1. Ibarra L, Espínola F, Echeverría M. Una prospección a la población de perros existente en las calles de Santiago de Chile. Avances en Ciencias Veterinarias 2006; 21: 33-9.
2. Amundson Romich J. Understanding zoonotic diseases.1st ed. Nueva York: Thomson Delmar Learning, 2008.
3. Larrieu EN. Prevención y control de la hidatidosis en el nivel local. Iniciativa Sudamericana para el control y vigilancia de la equinococosis quística/hidatidosis. Organización Panamericana de la Salud-OPS/OMS, Río de Janeiro. Serie de Manuales Técnicos, 2017, 18, p 56.
4. Bergagna H. Municipios no eutanásicos: perros y zoonosis. Desde la Patagonia, Difundiendo Saberes 2009; 6: 20-4.
5. Schiavini A, Narbaiza C. Estado de situación de los conflictos derivados de las poblaciones caninas en Tierra del Fuego. Informe realizado por solicitud del Comité de Emergencia Agroganadero y de Alerta Sanitaria de Tierra del Fuego. 2015; 37 pp. En: http://www.cadic-conicet.gob.ar/wp-content/uploads/2015/06/Conflictos-derivados-de-las-poblaciones-caninas-en-Tierra-del-Fuego-2015.pdf; consultado julio 2017.
6. Zunino M, De Francesco M, Kuruc J, Schweigmann N, Wisnivesky-Colli C, Jensen O. Contaminación por helmintos en espacios públicos de la provincia de Chubut, Argentina. Bol chil parasitol 2000; 55: 78-83.
7. Sánchez Thevenet P, Jensen O, Mellado I, et al. Presence and persistence of intestinal parasites in canine fecal material collected from the environment in the province of Chubut, Argentine Patagonia. Vet Parasitol 2003; 117: 263-9.
8. Sánchez Thevenet P, Ñancufil A, Oyarzo CM, et al. An eco-epidemiological study of contamination of soil with infective forms of intestinal parasites. Eur J Epidemiol 2004; 19: 481-9.
9. Soriano SV, Pierangeli NB, Roccia I, et al. A wide diversity of zoonotic intestinal parasites infects urban and rural dogs in Neuquén, Patagonia, Argentina. Vet Parasitol 2010; 167: 81-5.
10. Semenas L, Flores VR, Viozzi GP, Vázquez G, Pérez A, Ritossa L. Helmintos zoonóticos en heces caninas de barrios de Bariloche (Río Negro, Patagonia, Argentina). Revista Argentina de Parasitología 2014; 2: 22-7.
11. Caldas E, Casas N, Del Grande L, et al. Informe Equinococosis – N° 1 – Informe Epidemiológico en la Región de América del Sur – 2009-2014 PANAFTOSA Salud Pública Veterinaria 2015 – OPS/OMS. En: http://www.who.int/echinococcosis/resources/Inf_Epidem_Equinococosis-2009-2014.pdf?ua=1; consultado julio 2017.
12. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004; 17: 107-35.
13. Larrieu E, Seleiman M, Herrero E, et al. Vigilancia de la equinococosis quística en perros y niños en la provincia de Río Negro, Argentina. Rev Argent Microbiol 2014; 46: 91-7.
14. Guarnera EA. Hidatidosis en Argentina: carga de enfermedad. Organización Panamericana de la Salud (OPS). Buenos Aires: INEI, ANLIS Dr. Carlos G. Malbrán, Ministerio de Salud, 2009.
15. Acosta-Jamett G, Cleavelad S, Bronsvoort BM, Cunningham AA, Bradshaw H, Craig PS. Echinococcus granulosus infection in domestic dogs in urban and rural areas of the Coquimbo region, north-central Chile. Vet Parasitol 2010; 169: 117-22.
16. Casas N, Costas Otero S, Céspedes G, Sosa S, Santillán G. Detección de coproantígenos para el diagnóstico de echinococosis canina en la zona fronteriza de La Quiaca-Villazón. Rev Argent Microbiol 2013; 45: 154-9.
17. Larrieu E, Iriarte J, Zavaleta O. Aportes al conocimiento de la hidatidosis como zoonosis urbana. Rev Inst Med Trop Sao Paulo1988; 30: 28-31.
18. Larrieu E, Zanini F. Critical analysis of cystic echinococcosis control programs and praziquantel use in South America, 1974-2010. Rev Panam Salud Publica 2012; 31: 81-7.
19. Bingham GM, Larrieu E, Uchiumi L, et al. The economic impact of cystic echinococcosis in Río Negro province, Argentina. Am J Trop Med Hyg 2016; 94: 615-25.
20. Bendini M, Steimbreger N. Ocupaciones y movilidades en pueblos rurales de la Patagonia. Una mirada desde lo agrario. Mundo agr 2011; 12: 1-15.
21. Garibotti G, Zacharías D, Flores V, et al. Responsible ownership of dogs and human health in neighborhoods of San Carlos de Bariloche, Argentina Medicina (B Aires 2017; 77: 309-13.
22. Instituto Nacional de Estadísticas y Censos. Censo 2010. En: sig.indec.gov.ar/censo2010/; consultado septiembre 2017.
23. Blagburn, B. Internal parasites of cats and dogs. Diagnostic manual. Auburn: Novartis Animal Health, 2010.
24. Pierangeli N, Soriano S, Roccia I, et al. Usefulness and validation of a coproantigen test for dog echinococcosis screening in the consolidation phase of hydatid control in Neuquén, Argentina. Parasitol Int 2010; 59: 394-9.
25. R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing; 2015. R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. En: http://www.R-project.org/; consultado junio 2017.
26. Brusoni C, Fernández Canigia J, Lara J, Dezzotti A, Tamaño y estructura de la población canina en San Martín de los Andes (Neuquén). Analecta Vet 2007; 27: 11-23.
27. Morales MA, Varas C, Ibarra L. Caracterización demográfica de la población de perros de Viña del Mar, Chile. Arch med vet 2009; 41: 89-95.
28. Acosta-Jamett G, Cleaveland S, Cunningham A, Bronsvoort B. Demography of domestic dogs in rural and urban areas of the Coquimbo region of Chile and implications for disease transmission. Prev Vet Med 2010; 94: 272-81.
29. Vélez-Hernández L, Reyes-Barrera KL, Rojas-Almaráz D, Calderón-Oropeza MA, Cruz-Vázquez JK, Arcos-García JL. Riesgo potencial de parásitos zoonóticos presentes en heces caninas en Puerto Escondido, Oaxaca. Salud pública Méx 2014; 56: 625-30.
30. Trillo-Altamirano M, Carrasco AJ, Cabrera F. Prevalencia de helmintos enteroparásitos zoonóticos y factores asociados en Canis familiaris en una zona urbana de la ciudad de Ica, Perú. Parasit Latinoam 2003; 58: 136-41.
31. López J, Abarca K, Paredes P, Inzunza E. Intestinal parasites in dogs and cats with gastrointestinal symptoms in Santiago, Chile. Consideraciones en Salud Pública. Rev Med Chil 2006; 134: 193-200.
32. Torrecillas C, Mellado I, Resser C, et al. Parásitos de interés zoonótico y parasitosis intestinales humanas: situación y gestión de soluciones a escala local en una ciudad de Patagonia (Comodoro Rivadavia, Chubut, Argentina). Rev Argent Zoonosis Enferm Infecc Emerg 2014; 2: 35-7.
33. Kirkpatrick CE. Epizootiology of endoparasitic infections in pet dogs and cats presented to a veterinary teaching hospital. Vet Parasitol 1988; 30: 113-24.
34. Gracenea M, Gómez MS, Torres J. Prevalence of intestinal parasites in shelter dogs and cats in the metropolitan area of Barcelona (Spain). Acta Parasitol 2009; 54: 73-7.
35. Moro P, Schantz PM. Cystic echinococcosis in the Americas. Parasitol Int 2006; 55 Suppl: S181-6.
36. Moro PL, Lopera L, Cabrera M, et al. Short report: endemic focus of cystic echinococcosis in a coastal city of Peru. Amer J Trop Med Hyg 2004; 71: 327-9.
37. Merino V, Falcón N, Morel N, González G. Detección de coproantígenos de Echinococcus granulosus en canes de trabajadores de camales y comercializadores de vísceras en Lima metropolitana. Rev Pan Am Salud Publica 2017; 41: e10.
38. Farias LN, Malgor R, Cassaravilla C, Bragança C, De La Rue ML. Echinococcosis in Southern Brazil: efforts toward implementation of a control program in Santana do Livramento, Rio Grande do Sul. Rev Inst Med Trop Sao Paulo 2004; 46: 153-6.
39. P……………………………………………..érez A, Costa MT, Cantoni G, et al. Vigilancia epidemiológica de la equinococosis quística en perros, establecimientos ganaderos y poblaciones humanas en la provincia de Río Negro. Medicina (B Aires) 2006; 66: 193-200.
40. Souto MG, Sánchez Thevenet P, Basualdo Farjat J. Evaluation of the presence of Echinococcus granulosus sensu lato in the environment and in hosts in a region endemic for hydatidosis in the province of Chubut (Argentina). Vet Parasitol 2016; 6: 42-6.
41. Dirección de Estadísticas de la Municipalidad de San Carlos de Bariloche, 2016. En: us.qlikcloud.com/hub/user/56dd95499f51e412006d8b21/Dirección%20de; consultado septiembre 2017.
42. Beck K, Conboy C, Gilleard J, et al. Canadian guidelines for the treatment of parasites in dogs and cats. Canadian Parasitology Expert Panel, 2009, 32 pp. En: http://www.wormsandgermsblog.com/files/2008/03/CPEP-guidelines-ENGLISH1.pdf; consultado septiembre 2017.
43. Vaniscotte A, Raoul F, Poulle ML, et al. Role of dog behaviour and environmental fecal contamination in transmission of Echinococcus multilocularis in Tibetan communities. Parasitology 2011; 138: 1316-29.
– – – –
Clinical research is concerned with sick patients and thus with man and his diseases. But like other branches of science it uses the knowledge, techniques, and ideas of kindred disciplines. Many clinical research workers also use animals for experiments and for reasons that are obvious. But it must always be remembered that, so far as human disease is concerned, the first observation and the final proof, in so far as can ever be final, is made in man, there is no substitute. Thus if the relief of human suffering and the improvement of human society are the objects of medical research, observation and experiment in man are the lynchpin of the whole structure. This needs re-emphasis, because it is sometimes forgotten.
La investigación clínica se refiere a enfermos y por lo tanto al hombre y sus enfermedades. Pero, como otras ramas de la ciencia usa el conocimiento, técnicas e ideas de disciplinas relacionadas. Muchos investigadores clínicos también usan animales para sus experimentos, y por razones que son obvias. Pero se debe recordar siempre que, en cuanto a enfermedades humanas se refiere, la primera observación y la prueba final, tanto como alguna vez puede ser final, se hace en el hombre. Por consiguiente si el alivio del sufrimiento humano y la mejora de la sociedad son el objeto de la investigación médica, la observación y el experimento en el hombre son el eje de toda la estructura. Esto necesita re-enfatizarse, porque a veces se olvida.
George W. Pickering (1904-1980)
Clinical Research. En: Medical Research. Priorities and Responsibilities. Geneva: WHO, 1970, p 46
