MARÍA DE LOS ÁNGELES GARGIULO1, MARINA KHOURY2, GRACIELA GÓMEZ1, SEBASTIÁN GRIMAUDO1,
LORENA SUÁREZ1, MARÍA VICTORIA COLLADO1, JUDITH SARANO1
1Servicio de Inmunología, 2Estadística y Metodología de la Investigación, Instituto de Investigaciones Médicas Alfredo Lanari, Facultad de Medicina, Universidad de Buenos Aires, Argentina
Abstract Cut-off values for anti-dsDNA, anti-nucleosome and anti-C1q antibodies tests and for complementmediated hemolytic activity (CH50) were explored to identify patients with high risk of developing severe lupus nephritis (LN). Forty-one patients with confirmed systemic lupus erythematosus (SLE) were identified; their levels for the three antibodies and complement had been measured on a same serum sample. These patients were classified based on the presence of renal involvem ent; sixteen had active proliferative LN. With the cut-off values accepted in the laboratory for SLE diagnosis (anti-dsDNA > 100 UI/ml, anti-nucleosome > 50 U/ ml or CH50 < 190 UCH50%) no significant differences were found between patients with and without LN. Anti-C1q > 40 U/ml showed a statistically significant association with LN and had 80% of specificity. Cut-off values for LN identified by Receiver Operating Characteristic curves (ROC) were higher for anti-dsDNA (> 455 IU/ml) and anti-nucleosome (>107 U/ml), lower for CH50 (< 150 UCH50%) and, for anti-C1q (> 41 U/ml) coincided with the cut-off values accepted for SLE. Anti-C1q > 134 U/ml had a 92% of specificity, 56% of sensibility and was associated with a fifteen-fold increased risk of LN. The simultaneous presence of anti-nucleosome > 107 U/ml and anti-C1q > 134 U/ml was associated with a 27-fold higher probability for LN. According to these results, the cut-off values used to detect SLE activity could be inadequate to identify patients at high risk of severe LN.
Key words: systemic lupus erythematosus, lupus nephritis, immunologic tests
Resumen Valores de corte de pruebas inmunológicas para identificar pacientes con mayor riesgo de nefritis lúpica grave. Se exploraron valores de corte para los ensayos de anti-ADNdc, anti-nucleosoma, anti-C1q y complemento hemolítico total (CH50) capaces de identificar los casos con mayor riesgo de nefritis lúpica (NL) grave. Se seleccionaron 41 pacientes ≥ 16 años con lupus eritematoso sistémico (LES) confirmado que tenían titulados los niveles de los tres anticuerpos y CH50, en una misma muestra de suero. Fueron clasificados según presencia de compromiso renal; 16 presentaron formas proliferativas de NL activa. Con los valores de corte aceptados por el laboratorio para el diagnóstico de LES (anti-ADNdc > 100 UI/ml, anti-nucleosoma > 50 U/ml o un CH50 < 190 UCH50%) no se encontraron diferencias significativas entre casos con y sin NL. Un anti-C1q > 40 U/ml tuvo una especificidad del 80% y mostró una asociación estadísticamente significativa con NL. Al aplicar curvas Receiver Operating Characteristic (ROC) para NL, se identificaron valores de corte más altos para anti-ADNdc (> 455 IU/ml) y anti-nucleosoma (> 107 U/ml), más bajo para CH50 (< 150 UCH50%) y para el anti-C1q (> 41 U/ml) coincidió con el aceptado para diagnóstico de LES. Un anti-C1q > 134 U/ml presentó una sensibilidad del 56%, una especificidad del 92% y se asoció con quince veces más riesgo de NL. La presencia simultánea de anti-C1q > 134 U/ml y anti-nucleosoma > 107 U/ml se asoció 27 veces más riesgo de NL. De acuerdo a estos resultados los valores de corte empleados para actividad en pacientes con LES podrían resultar inadecuados para identificar pacientes con mayor riesgo de NL grave.
Palabras clave: lupus eritematoso sistémico, nefritis lúpica, pruebas inmunológicas
Received: 6-VI-2017 Accepted: 22-VIII-2018
Postal address: María de los Ángeles Gargiulo, Servicio de Inmunología, Instituto de Investigaciones Médicas Alfredo Lanari, Combatientes de Malvinas 3150, 1427 Buenos Aires, Argentina
e-mail: angegargiulo@hotmail.com
High levels of anti-double stranded DNA antibodies (anti-dsDNA) and low values of complement components are used as serological markers of SLE systemic activity in clinical practice. However, the value of these assays to identify a particular clinical manifestation of SLE is still controversial11-14.
The aim of the study was to explore cut-off values of assays that measure complement-mediated hemolytic activity (CH50), anti-dsDNA, anti-nucleosome and anti-C1q antibodies that would identify SLE patients with high risk of developing severe LN.
Materials and methods
Patients over 16 years old who fulfilled at least four of the American College of Rheumatology 1997 (ACR 1997) criteria for SLE classification15, with CH50, anti-dsDNA, anti-C1q and anti-nucleosome antibodies tests measured on a same serum sample at the Immunology Unit of Instituto de Investigaciones Médicas Alfredo Lanari (IDIM) were retrospectively identified. In only one patient anti-nucleosome test had not been performed. Patients without clinical record at the institution were excluded. The Institutional Review Board approved the study.
Demographic data, clinical manifestations, and disease activity defined according to Systemic Lupus Erythematosus Disease Activity Index Score (SLEDAI) criteria16 at the time of each serum sample were collected from medical records. Patients with SLEDAI more than 4 were considered with clinical activity.
Active LN was defined by 24-hour urine protein excretion > 0.5 g/day and/or active urinary sediment and/or an increase in serum creatinine levels of more than 25% from previous determinations17. Active urinary sediment was considered as the presence of hematic casts or > 5 red or white blood cells (RBC/WBC) in the absence of other alternative causes. Patients were classified according to the presence or not of severe forms of active LN. Severe LN were defined as proliferative forms of glomerulonephritis confirmed by biopsy as LN class III or IV or class V associated to class III or IV according to world Health Organization (WHO)18 histological classification. In LN patients, the serum sample obtained within the period of three months before renal biopsy and previous to start immunosuppressant treatment were chosen. Medical records of the subjects included in the group of patients without LN do not report any finding of clinical or laboratory manifestations of renal involvement. SLE patients with active LN but without confirmation by renal biopsy were excluded from the study.
At the IDIM Laboratory of Immunology, the CH50 test is measured by the method of Kent and Fife (normal range 190 to 270 UCH50%). All antibodies IgG- isotype are measured by ELISA with commercial kits (INOVA Diagnostics for anti-dsDNA, Generic Assays GmbH for anti-nucleosome antibodies and Buhlmann Laboratories AG for anti-C1q). The cut-off values provided by manufacturers for each kit are anti-dsDNA > 75 IU/ml, anti-nucleosome > 50 U/ml and anti-C1q >15 U/ ml. These kits were acquired by IDIM Laboratory in 2000 and previous to being used for the first time, cut-off values were calculated as the mean plus three standard deviations (mean+3SD) from a group of 50 healthy subjects, according with the manufacturer recommendation19-21. The IDIM Laboratory cut-off were for anti-dsDNA > 100 UI/ml, anti-nucleosome > 50 U/ml and anti-C1q > 40 U/ml.
To compare differences between two groups, Mann-Whitney U test was used for numerical variables and Chi square test, for proportions. Sensitivity, specificity and likelihood ratios to identify LN in SLE patients for anti-dsDNA, anti-nucleosome and anti-C1q antibodies and CH50 were calculated. Receiver Operating Characteristic (ROC) curves and Youden Index were applied to identify cut-off points. Area under the curve (AUC) was used to assess discrimination power of each test. To assess the strength of association between positive tests and renal involvement, odds ratios (OR) were calculated with 95% confidence intervals (95% CI). Statistical analysis was performed with Stata 11.0 and MedCalc. A p value < 0.05 was considered statistically significant.
Results
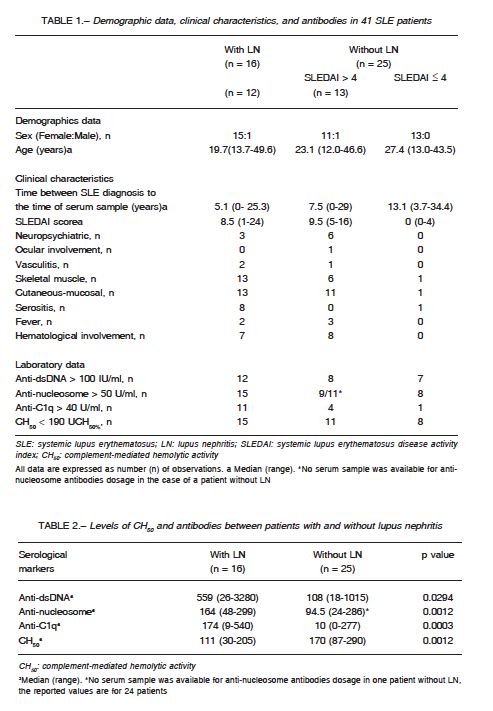
Serum samples from 41 SLE patients (2 men) were analysed. The median age at the time of diagnosis was 23 years (range 12-49) and the median time from the LES diagnosis to the serum sample was 10 years (range 0-34). In 16 patients with LN (12 class IV, 2 class V + III and 2 class III), the median values of creatinine and proteinuria were, respectively, 1.24 mg/dl (range 0.70-5.95) and 2.30 mg/24 hours (range 0.59- 5.95). In 25 patients without LN the median value of creatinine was 0.84 mg/dl (range 0.68-1.33) and 12 had SLEDAI more than 4. The sample description is presented in Table 1.
Statistically significant differences were found in serum levels of CH50 and antibodies (anti-dsDNA, anti-nucleosome and anti-C1q) between patients with and without LN (Table 2).
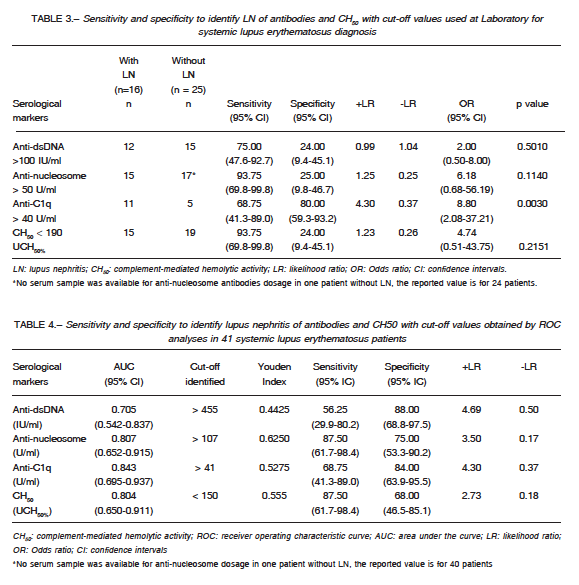
Table 3 shows the sensitivity and specificity of CH50 and anti-dsDNA, anti-nucleosome and anti-C1q tests to detect LN using cut-off values accepted at IDIM Laboratory. Anti-C1q was the only test with statistically significant association with renal involvement.
Table 4 shows the cut-off values, identified by ROC analyses, to detect LN in SLE patients. The cut-off values were > 455 IU/ml for anti-dsDNA, > 107 U/ml for anti-nucleosome, > 41U/ml for anti-C1q and < 150 UCH50% for CH50. These values were different from those use at IDIM in all tests except for the anti-C1q assay. It was observed that a cut-off value for anti-C1q > 134 U/ml had a high positive likelihood ratio (7.03), with lower sensitivity (56.25%, 95% CI: 29.9-80.2) and high specificity (92.00%, 95% CI: 4.0-99.0) for the detection of LN. Thus, it was decided to inform the following results using this cut-off value.
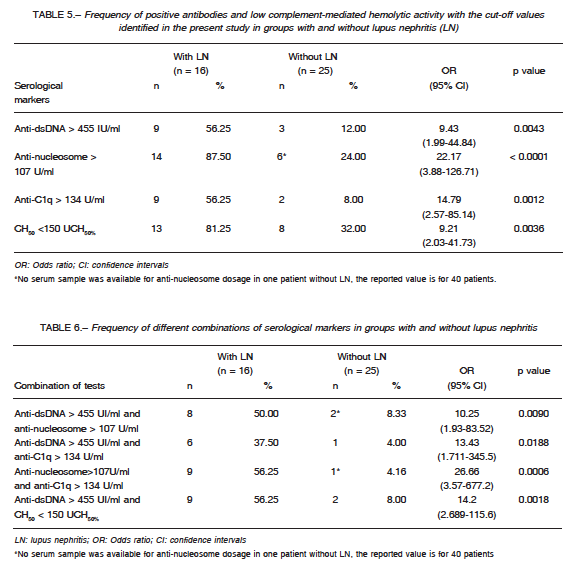
The frequency of positive antibodies and low CH50, using cut-offs identified by ROC analysis, in patients with and without LN is showed in Table 5. The four tests (anti-dsDNA > 455 IU/ml; anti-nucleosome > 107 U/ml; anti-C1q > 134 U/ml; CH50 <150 UCH50%), were statistically significant associated with the presence of LN.
Out of 14 patients with anti-dsDNA less than 455 IU/ ml; anti-nucleosome < 107 U/ml; anti-C1q < 134U/ml
and CH50 > 150 UCH50%, only one had LN. In contrast, out of 12 patients with three or more serological positive markers (anti-dsDNA > 455 IU/ml; anti-nucleosome > 107 U/ml; anti-C1q > 134U/ml) and/or CH50 < 150 UCH50%, 10 had LN. Table 6 shows the frequencies of different combinations of two tests in patients with and without LN. The simultaneous presence of anti-nucleosome > 107 U/ml and anti-C1q >134 U/ml was the combination with higher odds ratio to detect LN in SLE patients.
Discussion
Anti-dsDNA, anti-nucleosome, anti-C1q antibodies and complement activation, all serological markers of systemic activity, would play an important role in the inflammation and glomerular damage described in SLE patients22-26. In the present study, antibodies and CH50 levels had different median among patients with LN compared to patients without LN. These results are consistent with other studies that measured antibodies and complement levels in patients with active renal disease and proliferative forms of LN27-29.
When cut-off values accepted for SLE diagnosis at IDIM Laboratory were used, there were not statistically significant differences among anti-dsDNA, anti-nucleosome or CH50 in comparison between patients with and without LN. Anti-C1q antibody was the only one with significant statistically association to LN. These results are consistent with the anti-C1q antibody good performance to detect nephritis. Several authors proposed anti-C1q antibody as a serological marker of SLE with active renal involvement 8-10.
Although these immunological markers are not diagnostic tests for LN and do not replace renal biopsy, a contribution of this study was the identification of cut-off values to detect patients with high risk of severe LN different that those used to detect SLE activity. The cut-off values identified with ROC analysis to detect LN patients
were lower for CH50, higher for anti-dsDNA and anti-nucleosome, but was the same for anti-C1q. The cut-off values identified presented higher specificity and positive Likelihood Ratios (+LR) than those used to detect SLE activity. In this study, predictive values were not informed as they change in groups accordingly to the prevalence. Instead, LR were informed that allow to calculating posttest probability independently of prevalence.
Subjects with anti-C1q levels above 40 U/ml had eight times high risk of had LN. An anti-C1q value higher than > 134 U/ml had higher specificity and it was associated with a fifteen-fold increased risk for active kidney disease. These results suggest, in agreement with other studies, that the detection of anti-C1q antibodies alone or in combination with other serological markers of SLE activity could contribute useful information to identify patients at high risk of LN8-10, 30, 31.
Several studies proposed combining different serological markers to improve detection of patients at increased risk of proliferative LN28, 29, 39. Moroni et al28,29 described that the combination of anti-C1q and low complement was associated with active LN. Yang et al32 found that the simultaneous presence of anti-C1q and anti-dsDNA were associated with higher LN activity and poor renal outcome compared to only one or none of these antibodies. Orbai et al30 reported a strong association between combination of anti-C1q, anti-dsDNA and low complement with renal involvement. The present study showed low probability for LN when the combination of anti-dsDNA ≤ 455 IU/ml; anti-nucleosome ≤ 107 U/ml; anti-C1q ≤ 134U/ml and CH50 ≥ 150 UCH50% was present. The simultaneous detection of anti-nucleosome >107 U/ml and anti-C1q > 134U/ml, was associated with a 27-fold higher probability for LN. The combination of CH50 > 150 UCH50% and anti-dsDNA > 455 IU/ml showed a 14-fold higher probability for LN. This last observation is important since most laboratories may determine these two markers although the measurement of anti-nucleosome or anti-C1q may not be available.
This study has several limitations; one of them is the sample size because it was possible to identify only 41 SLE patients with determinations of the three antibodies and CH50 in the same serum sample. Prospective studies with a greater number of patients are required to analyze the consistency of this exploratory study. In addition, there was a lack of data about ethnic characteristics of the patients that might have some influence on the results of the antibodies and could explain differences with other studies.
Another important limitation is related with the technical diversity available to measure autoantibodies in serum sample. There is not universal standardized assay used by all laboratories to detect anti-dsDNA, anti-nucleosome, anti-C1q and CH50. Currently, there are guidelines for defining, establishing and verifying reference intervals in the clinical laboratory. These recommendations include specific and standardized procedures that can be used to establish and verify reliable reference intervals between different laboratory33, for assessment of the diagnostic accuracy of laboratory tests using ROC analysis34 or for evaluation of qualitative test performance35.
In a multicenter study30 that measured anti- C1q with purified collagenous C1q fragments as antigen in the ELISA technique, the cut-off value was defined as < 16 AU/l based on analysis of 96 healthy blood donors. Although, this cut-off value was a different and no comparable from those of this study, also reported an anti-C1q specificity of 85% for the detection of NL. Like Mok et al.36, the present study measured anti-dsDNA, anti-nucleosome and anti- C1q antibodies in the same serum sample and reported that anti-C1q was the most specific test. However, they found that anti-dsDNA was more sensitive than anti-nucleosome antibodies to detect LN. The discrepancy between studies results could be a consequence of the nature of the antigens and types of assays used in anti-nucleosome tests, which may determine cross-reactions with anti-dsDNA. Nucleosomes are repeating subunits of chromatin consisting of DNA molecules coiled around a core of basic histone and non-histone proteins13. The term “anti-nucleosome antibodies” includes various antibodies of different specificities that may react with histones, histone variants, modified histones, non-histone proteins, dsDNA structures, or diverse conformational determinants unique to the complex structure in chromatin. There is not a single uniform criterion to define the antigenic target of anti-nucleosome antibodies, so it is difficult to standardize laboratory tests and the use of this antibody in clinical practice is questionable5, 37. Some authors have described the advantages of using as antigen DNA-loaded nucleosomes (anti-dsDNA-NcX) ELISA test38, 39 .
The present study explored the possibility to detect quickly and with non-invasive methods patients with high risk of LN. The results suggest that anti-dsDNA, anti-nucleosome, anti-C1q and CH50 cut-off values used for the detection of SLE activity could be inadequate to identify patients with a high risk of severe LN. Each center laboratory would consider set up cut-off values different from those established for SLE diagnosis to evaluate the risk of severe renal involvement and select their best combination of serological markers, including anti-C1q test, if possible, due to its high specificity.
Acknowledgments: The authors would like to thank to Dr. Viviana Tagliaficci and Dr. Manuel Buhl for their assistance in developing the project and Mr. Sebastián de la Mata for his technical help.
Conflict of interests: None to declare
References
1. Dooley MA. Clinical and laboratory features of lupus nephritis. In: Wallace DJ and Hahn BH (eds) Dubois’s Lupus Erythematosus. 7th ed. Philadelphia: Lippincott Williams & Williams, 2007, 1112-30.
2. Hahn BH, Mc Mahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res 2012; 64: 797-808.
3. Cervera R, Khamastha MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10- year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003; 82: 299-308.
4. Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational latin american prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 2004; 83: 1-17.
5. Gómez-Puerta JA, Burlingame RW, Cervera R. Anti-chromatin (anti-nucleosome) antibodies: diagnostic and clinical value. Autoimmun Rev 2008; 7: 606-11.
6. Yin S, Ru-Lin J, Lei H, et al. Role of antinucleosome antibody in the diagnosis of systemic lupus erythematosus. Clin Immunol 2007; 122: 115-20.
7. Bizzaro N, Villalta D, Giavarina D, Tozzoli R. Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis. Autoimmun Rev 2012; 12: 97-106.
8. Marto N, Bertolaccini ML, Calabuig E, Hughes GR, Khamashta MA. Anti-C1q antibodies in nephritis: correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosus. Ann Rheum Dis 2005; 64: 444-8.
9. Yin Y, Wu X, Shan G, Zhang X. Diagnostic value of serum anti-C1q antibodies in patients with lupus nephritis: a meta-analysis. Lupus 2012; 21: 1088-97.
10. Akhter E, Burlingame RW, Seaman AL, Magder L, Petri M. Anti-C1q antibodies have higher correlation with flare of lupus nephritis than other serum markers. Lupus 2011; 20: 1267-74.
11. Wasmuth JC, Grun B, Terjung B. ROC analysis comparison of three assays for the detection of antibodies against double-stranded DNA in serum for the diagnosis of systemic lupus erythematosus. Clin Chem 2004; 50: 2169-71.
12. Gladman DD, Urowitz MB, Keystone EC. Serologically active clinically quiescent systemic lupus erythematosus: discordance between clinical and serologic features. Am J Med 1979; 66: 2010-5.
13. Heidenreich U, Mayer G, Herold M, Klotz W, Stempfl Al- Jazrawi K, Lhotta K. Sensitivity and specificity of autoantibody test in the differential diagnosis of lupus nephritis. Lupus 2009; 18: 1276-80.
14. Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus 2004; 13: 290-7.
15. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725.
16. Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29: 288-291.
17. Sociedad Argentina de Reumatología y Sociedad Argentina de Nefrología. Primer consenso sobre diagnóstico y tratamiento de la nefropatía lúpica. Rev Arg Reumatol 2011; 22: 6-37.
18. Churg J, Bernstein J, Glassock R. Renal disease: classification and atlas of glomerular diseases. New York, NY: Igaku-Shoin, 1995.
19. Anti-C1q insert. En: https://www.buhlmannlabs.ch/wp-content/uploads/2015/03/ek-ac1qa_ifu-ce_13-01-31.pdf; consultado noviembre 2017.
20. Anti-dsDNA insert. En: https://www.inovadx.com/store/categories/quanta-liter-elisa/2; consultado noviembre 2017.
21. Anti nucleosome insert. En: https://www.genericassays. com/produkte/eia; consultado noviembre 2017.
22. Berden JH. Lupus nephritis. Kidney Int 1997; 52: 538-58.
23. Hahn BH. Antibodies to DNA. N Engl J Med 1998; 338: 1359-68.
24. Gatto M, Iaccarino L, Ghirardello A, Punzi L, Doria A. Clinical and pathologic considerations of the qualitative and quantitative aspects of lupus nephritogenic autoantibodies: A comprehensive review. J Autoimm 2016; 69: 1-11.
25. Van der Vlag J, Berden J.H. Lupus nephritis: role of antinucleosome autoantibodies. Semin Nephrol 2011; 31: 376-89.
26. Flierman R, Daha MR. Pathogenic role of anti-C1q autoantibodies in the development of lupus nephritis-a hypothesis. Mol Inmunol 2007; 44: 133-8.
27. Živković V, Stanković A, Cvetković T, et al. Anti-dsDNA, anti-nucleosome and anti-C1q antibodies as disease activity markers in patients with systemic lupus erythematosus. Srp Arh Celok Lek 2014; 42: 431-6.
28. Moroni G, Radice A, Giammarresi G, et al. Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis 2009; 68: 234-7.
29. Moroni G, Quaglini S, Radice A, et al. The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsy. J Immunol Res 2015; ID 1069042: 1-8.
30. Orbai A, Truedsson L, Sturfelt G, et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus 2015: 24: 42-9
31. Gargiulo M, Gomez G, Khoury M, et al. Anti-C1q antibodies related to active nephritis in patients with systemic lupus erythematosus. Medicina (B Aires) 2015; 75: 23-8.
32. Yang X, Tan Y, Yu F, Zhao M. Combination of anti-C1q and anti-dsDNA antibodies is associated with higher renal disease activity and predicts renal prognosis of patients with lupus nephritis. Nephrol Dial Transplant 2012; 27: 3552–9.
33. Horowitz G, Altaie, S, Boyd J, et al. Defining, establishing and verifying reference intervals in the clinical laboratory; approved guideline-third edition. Clinical and Laboratory Standards Institute CLSI document EP28-A3c. Wayne, PA; 2008.
34. Kroll M, Biswas B, Budd J, et al. Assessment of the diagnostic accuracy of laboratory tests using receiver operating characteristic curves; approved guideline-second edition. Clinical and Laboratory Standards Institute CLSI document EP24-A2. Wayne, PA; 2011.
35. Garrett P, Lasky F, Meier K. User protocol for evaluation of qualitative test performance; aproveed quidelines – second edition. Clinical and Laboratory Standards Institute CLSI document EP12-A2. Wayne, PA; 2008.
36. Mok C, Ho L, Leung H, Wong L. Performance of anti-C1q, antinucleosome, and anti-dsDNA antibodies for detecting concurrent disease activity of systemic lupus erythematosus. Translational Research 2010; 156: 320-5.
37. Revkig O L, van der Vlag J, Seredkina N. Antinucleosome antibodies. A clinial reflection on their specificies and diagnostic impact. Arthritis Rheum 2014; 5: 1061-9.
38. Biesen R, Dähnrich C, Rosemann A, et al. Anti-dsDNA-NcX ELISA: dsDNA-loaded nucleosomes improve diagnosis and monitoring of disease activity in systemic lupus erythematosus. Arthritis Res Ther 2011; 13 R26.
39. Dieker J, Schlumberger W, McHugh N, Hamann P, van der Vlag J, Berden BH. Reactivity in ELISA with DNA-loaded nucleosomes in patients with proliferative lupus nephritis. Mol Immunol 2015; 68: 20-4.
– – – –
Las dificultades también pasan, como todo pasa, sin dificultad.
Antonio Porchia (1886-1968)
Voces. 2da. Edición. Buenos Aires: Hachette, 1969, p 62
