SOLEDAD SOSA, KARINA DANILOWICZ, LEONARDO F. L. RIZZO
División Endocrinología, Hospital de Clínicas José de San Martín, Universidad de Buenos Aires, Argentina
Resumen Diversos mecanismos adaptativos se ponen en marcha para sostener las funciones orgánicas vitales en el paciente crítico. El eje hipotálamo-hipófiso-adrenal tiene un papel clave en la respuesta al estrés al regular el metabolismo, la función cardiovascular y la respuesta inmune. Esta revisión tiene por objetivos analizar los cambios fisiopatológicos que se producen en el eje adrenal durante la enfermedad crítica, reconocer las limitaciones de los métodos diagnósticos y definir indicaciones de tratamiento de reemplazo corticoideo en este contexto. El concepto de insuficiencia adrenal relativa debe ser descartado y no se recomienda el test de estímulo con cosintropina para diagnóstico de insuficiencia adrenal durante enfermedad crítica ni para definir la necesidad de tratamiento.
Palabras clave: cortisol, metabolismo, insuficiencia adrenal, enfermedad crítica
Abstract After a stressful event, adaptative mechanisms are carried out to support vital functions. Hypothalamic-pituitary-adrenal axis plays a key role in stress response regulating metabolism, cardiovascular function and immune system. This review addresses pathophysiological changes of the adrenal axis during critical illness, recognizing limitations of methods applied for its evaluation in this special context and defining indications for corticosteroid replacement in critically ill patients. The concept of relative adrenal insufficiency should be abandoned; cosyntropin stimulation test should not be performed for diagnosis of adrenal insufficiency in critical illness nor for establishing the need of treatment.
Key words: cortisol, metabolism, adrenal insufficiency, critical illness
Postal address: Soledad Sosa, Hospital de Clínicas José de San Martín, Universidad de Buenos Aires, Av. Córdoba 2351 5° piso, Sala 2, 1120 Buenos Aires, Argentina
e-mail: drasoledadsosa@yahoo.com.ar
• Diagnosis of adrenal insufficiency during critical illness should consider the past medical history, current medications, the pathophysiological changes in hypothalamicpituitary-adrenal axis (decreased cortisol catabolism, reduced binding proteins, increased distribution volume, differential glucocorticoid receptor expression, etc.) and the limitations of diagnostic methods in this context.
• Diagnosis relays on serum total cortisol levels according to binding protein levels. Cosyntropin stimulation test should be avoided in critically ill patients.
• Treatment with hydrocortisone, if needed due to adrenal insufficiency, should not exceed 200 mg daily and be tapered as soon as possible to the physiological dose.
Stress is an unexpected event that alters homeostasis.
When caused by severe illness or traumatism, surgery or extended burns, stress threatens life1. Many times, management in intensive care unit is mandatory, due to severe respiratory, cardiovascular and/or neurological derangement, conditioning the individual to be defined as a critically ill patient.
In this scenario, several adaptative mechanisms are carried out to support vital functions and to recover homeostasis.
Main objectives are assuring perfusion and energy availability for crucial tissues through maintenance of blood pressure and stimulus of gluconeogenesis and glycogenolysis, respectively1-3.
Adaptative mechanisms such as sympathetic and hypothalamic-pituitary-adrenal axis (HPAA) activation exert immediate effects. While somatotroph activation and glucagon secretion occur in a deferred manner1, 4.
Cortisol plays a key role in stress response: it contributes to energy supply by increasing catabolism and delaying anabolism. Additionally, it regulates immune system, cardiovascular function and hydroelectrolytic balance1.
This review addresses pathophysiological changes of HPAA during critical illness, recognizing limitations of methods applied for HPAA evaluation in this special context and defining indications for corticosteroid replacement in critically ill patients.
Hypothalamic-pituitary-adrenal axis regulation in critical illness
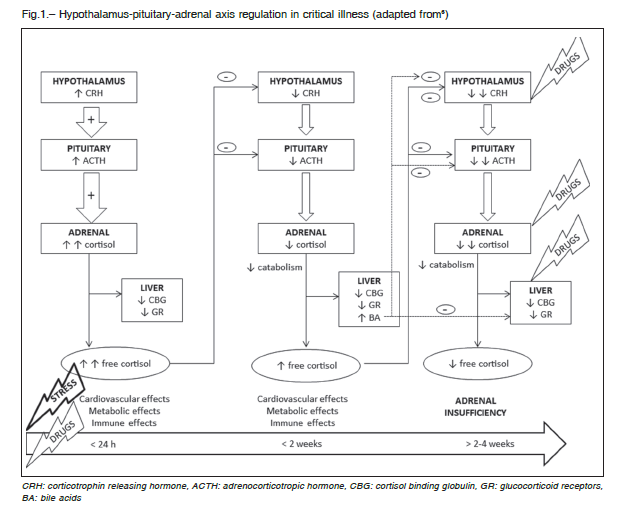
Activation of hypothalamic-pituitary-adrenal axis (HPAA) in response to critical illness is an immediate event after injury. Hypothalamic corticotropin releasing hormone (CRH) stimulates adrenocorticotropic hormone (ACTH) secretion from the pituitary. The later induces cortisol production in adrenal glands and as long as cortisol cannot be stored in the adrenals, its availability relays on synthesis in response to stress5.
Subsequently, although cortisol production declines, serum free cortisol levels increase6-8, because levels and affinity of cortisol binding globulin (CBG) decrease9-11.
Thus, cortisol bioavailability raises and triggers hypothalamic- pituitary feedback, leading to diminished CRH and ACTH levels and pulsatility 24 hours after injury6, 12.
Concurrently, in this second phase, level and activity of hepatic and renal metabolizing enzymes decrease, driving the prolongation of cortisol half-life6, 13, 14. This explains how serum free cortisol levels remain elevated (5 to 7 times normal), despite reduction in cortisol production6,12, 15-17.
Decreased cortisol catabolism becomes an energetically inexpensive mechanism of maintaining adequate levels of cortisol. Limitation of anabolic processes is crucial while low energy availability exists. However, prolonged low ACTH levels causes structural and functional adrenocortical impairment18, culminating in adrenal insufficiency after 2 to 4 weeks (Fig. 1)1, 18-20.
Another hypothesis concerning adrenal ACTH-independent stimulation during stress, argues involvement of adrenergic, immune mediators (interleukins 1 and 6, tumor necrosis factor), adipokines and endothelial neuropeptides21, 22.
Cortisol rhythm in critically ill patients lacks of the physiological evening reduction, the normal suppression after dexamethasone administration and shows exaggerated response to CRH, characteristics that assimilate it to Cushing’s syndrome23-25.
Finally, the need of increased cortisol production during critical illness for survival is uncertain26. In fact, there is no cortisol threshold below which mortality has been augmented21.
Variables to consider in critical illness
Cortisol kinetics in the bloodstream
In basal conditions, considering a total cortisol of 15 mcg/ dl, 90% of cortisol binds to carrier globulins (75% to CBG -11 mcg/dl- and 15% to albumin -2.5 mcg/dl-). The remaining 10% corresponds to serum free cortisol (1.5 mcg/dl) that is readily available for biologic actions6.
In critically ill patients, these proportions differ due to decreased CBG and albumin levels. Considering total cortisol of 15 mcg/dl, cortisol binding to CBG represents approximately 58% (8.6 mcg/dl) and to albumin 11% (1.7 mcg/dl), whereas serum free cortisol raises to 31% (4.7 mcg/dl)23.
Cortisol changes exhibit variability among individuals depending on sex, heterogeneity and duration of subjacent critical illness, hydration state, protein levels, polymorphism of glucocorticoid receptors, CRH and ACTH levels and activity of metabolizing enzymes, in addition to methodological variability of cortisol measurement21.
It is important to recall that hepatic inflammation induces protein synthesis, including CBG that can raise serum total cortisol as opposed to critical illness. As a consequence, cortisol levels increase even in this last scenario27.
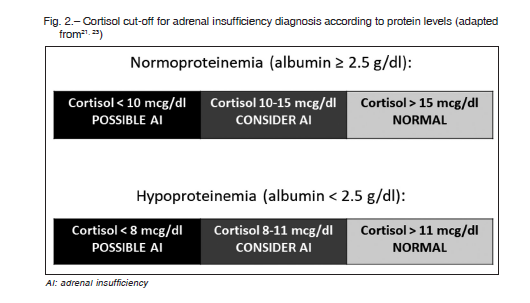
Due to the impossibility of CBG measurement in clinical practice, albumin is a useful subrogate marker for protein levels. Albumin levels lower than 2.5 g/dl indicate a hypoproteinemic state. Cortisol cut-off is defined accordingly for adrenal nsufficiency definition in critical illness: for albumin level ≥ 2.5 g/dl, adrenal sufficiency is considered when serum cortisol is > 15 mcg/dl and insufficiency if cortisol is < 10 mcg/dl. Whereas if albumin level is < 2.5 g/dl, adrenal sufficiency is considered with cortisol level > 11 mcg/dl and insufficiency when cortisol is < 8 mcg/dl17,28 (Fig. 2).
Furthermore, there is still no consensus regarding levels of serum free cortisol considered normal in critical illness. Levels of cortisol during major stress are supposed to be similar to those reached during cosyntropin stimulation test. Therefore serum free cortisol levels in critically ill patients are proposed to be >1.8 mcg/dl23.
Cortisol metabolism
There are different pathways to metabolize cortisol in renal and hepatic tissues. The renal pathway is mediated by the 11β-hydroxysteroid deshydrogenase type 2, which converts cortisol to the inactive form cortisone. Activity of this enzyme is decreased in critical illness (approximately by 50%), diminishing cortisol catabolism14.
In the liver, different enzymatic subgroups with 5-reductase activities (α y β) convert cortisol to inactive metabolites through tetrahydroxylation (5α- y 5β-tetrahydrocortisol).
These enzymes have reduced activity in critically ill patients, contributing to decreased cortisol breakdown12,29.
Globally, cortisol catabolism in critical illness is estimated to be 47% lesser than in healthy individuals.
Moreover, reduced enzyme activity is not the only mechanism to decrease cortisol metabolism but also reduced enzyme synthesis, demonstrated by low mARN and protein levels12.
Glucocorticoid receptors
There are two isoforms of glucocorticoid receptors (GR) generated through alternative splicing. Alpha-GR (α-GR) is the main isoform and has mostly genomic actions, while beta-GR (β-GR) has negative dominant effect, counteracting α-GR actions6.
Critical illness induces a change in GR expression, favoring the β-GR isoform. This phenomenon explains the glucocorticoid resistance postulated for critical illness30.
Hepatic GR activated by cortisol can regulate enzyme action and diminish its own catabolism31. Another important effect mediated by hepatic GR during critical illness is glucose homeostasis32-34.
During sepsis, GR are partially downregulated. Higher levels of cortisol, as part of high doses glucocorticoid treatment can further suppress hepatic GR, leading to hepatic inflammation, injury and increasing mortality31.
Bile acids
Besides its classical role in lipid absorption, bile acids have crucial functions in regulation of inflammation and endocrine homeostasis. Bile acids, like steroid hormones, are derivative products from cholesterol. Both metabolic pathways share enzymes and steps6.
For instance, the principal enzyme for cortisol catabolism, the 5-reductase, is pivotal for bile acids synthesis34. Subsequently, enzymatic feedback due to bile acids increase during critical illness (10 times above normal), determinates diminished levels and activity of the enzymes and consequently, diminished cortisol catabolism12, 29.
Bile acids interact with multiple nuclear receptors including GR35. Given the high levels of bile acids in the context of critical illness36, they are also able to cross the central nervous system barrier and inhibit HPAA at the hypothalamic level37.
Possible scenarios in critically ill patients
Critical illness can affect individuals with previous diagnosis of adrenal insufficiency, either primary or secondary.
On the other side, an unknown diagnosis of adrenal insufficiency due to lack of recognition or recent development of the disease, can be associated with adrenal hemorrhage, cranial traumatism, pituitary tumor or apoplexy38.
Adrenal insufficiency can be triggered by drugs that interfere cortisol synthesis (e.g. etomidate, ketoconazole) or enhance its catabolism (e.g. phenobarbital, phenytoin, rifampicin), agents that inhibit central regulation (e.g. opioids) or due to abrupt withdrawal of glucocorticoids or drugs with agonist effects (e.g. megestrol). Anticoagulants can precipitate adrenal hemorrhage and consequently, primary adrenal insufficiency. These situations should be taken into account while designing the clinical record23.
Relative adrenal insufficiency or critical illness-related corticosteroid insufficiency (CIRCI)
The concept was proposed by Rothwell in 199139. It is defined as the inability of reaching an increment in cortisol > 9 mcg/dl during cosyntropin stimulation test during critical illness. The author suggested the prognostic relevance in patients with septic shock as marker of increased mortality.
It has been applied thereafter as indicator for glucocorticoid replacement need. On this matter, Annane et al, evaluated 299 patients with septic shock classified according to cosyntropin stimulation test response40. Those who failed to respond (cortisol increase < 9 mcg/dl) were treated with hydrocortisone 50 mg every 6 hours associated with fludrocortisone 100 mcg daily. They reported that the treated group showed benefits in terms of mortality and shorter time to extubation. However, this study was hardly questioned as detailed below.
Evidence that refutes the existence of relative adrenal insufficiency or critical illness-related corticosteroid insufficiency (CIRCI)
The most sensitive issue is that pivotal studies have serious clinical and methodological limitations (treatment with etomidate in the responder group –a known steroidogenesis inhibitor–, high mortality in the placebo group and statistical methods used to report outcomes)21 added to the point of not considering physiological HPAA variations during critical illness.
There is opposed evidence. Widmer and cols. demonstrated that patients under major surgical stress with favorable outcome, showed a response to cosyntropin stimulation test < 9 mcg/dL, like one third of non-stressed healthy controls41.
The cosyntropin stimulation test, validated in nonstressed healthy population, lacks of reproducibility in critically ill patients, especially in those with septic shock26, 42.
Additionally, cortisol response in a HPAA maximally stimulated due to critical illness is expected to be reduced21.
Lower levels of carrier globulins in critical illness condition the elevation of cortisol in response to stimulus: critically ill patients with near normal protein levels showed greater cortisol response than patients with hypoproteinemia17, 23. Furthermore, larger distribution volume adds variability1, 6.
Diminished cortisol catabolism also impacts on reducing response to cosyntropin stimulation test12, 19. Elevated cortisol levels, decreased ACTH in response to feedback and adrenal tropism can influence test results21.
Some patients with partial central adrenal insufficiency can have a normal response to 250 mcg of cosyntropin and the 1 mcg test is not supported in this scenario23.
Thus, it seems that cosyntropin stimulation test is not an appropriate test for evaluating HPAA in the critical illness context.
Evaluation of hypothalamic-pituitary-adrenal axis function during critical illness
Considering the alterations in cortisol regulation and metabolism and carrier protein levels, interpretation of cortisol levels in critically ill patients differs from healthy individuals.
These limitations can lead to misinterpretation of the state of critically ill patients regarding adrenal sufficiency21.
The prevalence of adrenal sufficiency in critically ill patients has been reported between 0 and 77%. However, it is difficult to establish owing to the limitations of the evaluating tools, the differences among populations included in the studies and the diagnosis criteria applied43.
A more reliable tool would be the measurement of serum free cortisol, nevertheless it is not available in clinical practice and cut-off values have not been established for critically ill patients. Salivary free cortisol has good correlation with serum free cortisol and reflects its changes rapidly. Notwithstanding, getting a sample can be challenging in critically ill patients23.
As previously stated, cosyntropin stimulation test is not a valid test in critically ill patients, due to poor reproducibility in the context of high distribution volume (40% larger than normal) and decrease CBG levels among other factors8, 12.
These caveats also apply to the measurement of total cortisol, but thus far seems to be the most appropriate method for evaluating HPAA, taking into account the limitations in each case (protein levels, treatments that interfere synthesis, action, catabolism, etc.). As previously mentioned, due to the lack of circadian rhythm, random cortisol can be useful for diagnosis21 (Figure 2).
No other dynamic tests like insulin hypoglycemia, metyrapone test or CRH test are approved in critical illness25.
Treatment
Since half-life of cortisol is prolonged in critical illness, actual recommendations, based in guidelines published in 201744, of doses ranging 200-400 mg of hydrocortisone per day seem to be excessive and long-term consequences are unknown6.
Under septic shock conditions, these doses have shown to reduce duration of vasoactive drug need and ventilation, due to anti-inflammatory effects, without a clear reduction of mortality40, 45-47. In fact, long-term use of high doses of glucocorticoids causes hepatic glucocorticoid receptor suppression with increasing inflammation and mortality31. The need and modality of treatment in the acute phase is still controversial, given the disparity of the data45, 46.
The newest meta-analyses confirm the minimal or absent mortality benefit of glucocorticoid treatment in patients with septic shock. Rygård et al included 7297 patients of 22 studies and found no improvement in short-term or long-term mortality in patients treated with glucocorticoids compared to placebo (relative risk of 0.98, 95% confidence interval 0.89-1.08 and 0.96, 0.91-1.02, respectively) but only a shorter duration of shock, mechanical ventilation and intensive care unit stay48. Rochwerg et al. found similar results in a meta-analysis of 42 studies including 10 194 patients49. Both groups report greater frequency of adverse events in the treated group (hypernatremia, hyperglycemia).
In those critically ill patients without septic shock whose cortisol levels suggest adrenal insufficiency, the proposed treatment is hydrocortisone 25 mg every 6 hours and rapidly decrease of the dose according to clinical outcomes.
In the context of primary adrenal insufficiency, fludrocortisone 50-100 mcg should be added when doses of hydrocortisone are adjusted below 50 mg daily. In patients with septic shock, doses of hydrocortisone should be doubled (50 mg every 6 hours) followed by the same approach of rapid tapering of the dose according to clinical response23.
On the other hand, the prolongation of critical illness beyond 4 weeks accompanied by a decrease in cortisol levels, is probably the time for starting physiologic replacement therapy, if not started before, because of persistent low ACTH levels that lead to structural and functional impairment of adrenal cortex8.
To conclude, diagnosis of adrenal insufficiency during critical illness can be troublesome. A high suspicion level should be present for patients at risk of adrenal insufficiency and the leading cause should always be assessed.
Considering limitations of diagnostic methods in critically ill patients is of outmost importance. The cosyntropin stimulation test has no validity and is not needed for diagnosis.
Serum free cortisol is desirable but not available in clinical practice. Hence, serum cortisol is considered and interpreted according to carrier proteins.
The treatment with hydrocortisone, if needed, should not exceed 200 mg daily and be tapered as soon as possible to the physiological dose.
We discourage the concept of relative adrenal insufficiency or CIRCI and its treatment. Alternatively, we propose the concept of transient adaptative glucocorticoid resistance induced by stress. Although there is no method to evaluate glucocorticoid sensibility, some evidence can support an increase in resistance: increased cortisol bioavailability (through cleavage of carrier proteins or decreased catabolism), differentiated expression of glucocorticoid receptors, among others.
Conflicts of interest: None to declare
References
1. Téblick A, Langouche L, Van Den Berghe G. Anterior pituitary function in critical illness. Endocr Connect 2019;
8: R131-43.
2. Yang S, Zhang L. Glucocorticoids and Vascular Reactivity. Curr Vasc Pharmacol 2004: 2: 1-12.
3. Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011; 60: 1500-10.
4. Van Den Berghe G, De Zegher F, Bouillon R. Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab 1998; 83: 1827-34.
5. Vermes I, Beishuizen A, Hampsink RM, Haanen C. Dissociation of plasma adrenocorticotropin and cortisol levels in critically Ill patients: Possible role of endothelin and atrial natriuretic hormone. J Clin Endocrinol Metab 1995; 80: 1238-42.
6. Téblick A, Peeters B, Langouche L, Van den Berghe G. Adrenal function and dysfunction in critically ill patients. Nat Rev Endocrinol 2019; 15: 417-27.
7. Henley D, Lightman S, Carrell R. Cortisol and CBG – Getting cortisol to the right place at the right time. Pharmacol Ther 2016; 166: 128-35.
8. Peeters B, Meersseman P, Vander Perre S, et al. Adrenocortical function during prolonged critical illness and beyond: a prospective observational study. Intensive Care Med 2018; 44: 1720-9.
9. Nenke MA, Rankin W, Chapman MJ, et al. Depletion of high-affinity corticosteroid-binding globulin corresponds to illness severity in sepsis and septic shock; Clinical implications. Clin Endocrinol (Oxf) 2015; 82: 801-7.
10. Meyer EJ, Nenke MA, Rankin W, et al. Total and highaffinity corticosteroid-binding globulin depletion in septic shock is associated with mortality. Clin Endocrinol (Oxf) 2019; 90: 232-40.
11. Chan WL, Carrell RW, Zhou A, Read RJ. How changes in affinity of corticosteroid-binding globulin modulate free cortisol concentration. J Clin Endocrinol Metab 2013; 98: 3315-22.
12. Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med 2013; 368: 1477-88.
13. Tomlinson JW, Walker EA, Bujalska IJ, et al. 11β-Hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr Rev 2004; 25: 831-66.
14. Tomlinson JW, Stewart PM. Cortisol metabolism and the role of 11β-hydroxysteroid dehydrogenase. Best Pract Res Clin Endocrinol Metab 2001; 15: 61-78.
15. Boonen E, Van Den Berghe G. Cortisol metabolism in critical illness: Implications for clinical care. Curr Opin Endocrinol Diabetes Obes 2014; 21: 185-92.
16. Boonen E, Van Den Berghe G. Mechanisms in endocrinology: New concepts to further unravel adrenal insufficiency during critical illness. Eur J Endocrinol 2016; 175: R1-R9.
17. Hamrahian AH, Oseni TS, Arafah BM. Measurements of Serum Free Cortisol in Critically Ill Patients. N Engl J Med 2004; 350: 1629-38.
18. Boonen E, Langouche L, Janssens T, et al. Impact of duration of critical illness on the adrenal glands of human intensive care patients. J Clin Endocrinol Metab 2014; 99: 4214-22.
19. Boonen E, Van Den Berghe G. Endocrine responses to critical illness: Novel insights and therapeutic implications. J Clin Endocrinol Metab 2014; 99: 1569-82.
20. Barquist E, Kirton O. Adrenal insufficiency in the surgical intensive care unit patient. J Trauma 1997; 42: 27-31.
21. Hamrahian AH, Fleseriu M. Evaluation and management of adrenal insufficiency in critically ill patients: Disease state review. Endocr Pract 2017; 23: 716-25.
22. Kanczkowski W, Alexaki VI, Tran N, et al. Hypothalamopituitary and immune-dependent adrenal regulation during systemic inflammation. Proc Natl Acad Sci U S A 2013; 110: 14801-6.
23. Arafah BM. Review: Hypothalamic pituitary adrenal function during critical illness: Limitations of current assessment methods. J Clin Endocrinol Metab 2006; 91: 3725-45.
24. Peeters B, Langouche L, Van Den Berghe G. Adrenocortical stress response during the course of critical illness. Compr Physiol 2018; 8: 283-98.
25. Reincke M, Allolio B, Wurth G, Winkelmann W. The hypothalamic- pituitary-adrenal axis in critical illness: Response to dexamethasone and corticotropin-releasing hormone. J Clin Endocrinol Metab 1993; 77: 151-6.
26. Loriaux DL, Fleseriu M. Relative adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes 2009; 16: 392-400.
27. Barle H, Hammarqvist F, Westman B, et al. Synthesis rates of total liver protein and albumin are both increased in patients with an acute inflammatory response. Clin Sci (Lon) 2006; 110: 93-9.
28. Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003; 348: 727-34.
29. McNeilly AD, Macfarlane DP, Emmett O’Flaherty E, et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J Hepatol 2010; 52: 705-11.
30. Guerrero J, Gatica HA, Rodríguez M, Estay R, Goecke IA. Septic serum induces glucocorticoid resistance and modifies the expression of glucocorticoid isoforms receptors: A prospective cohort study and in vitro experimental assay. Crit Care 2013; 17: R107.
31. Jenniskens M, Weckx R, Dufour T, et al. The Hepatic Glucocorticoid Receptor Is Crucial for Cortisol Homeostasis and Sepsis Survival in Humans and Male Mice. Endocrinology 2018; 159: 2790-2.
32. Szabo G, Romics L Jr, Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis 2002; 6: 1045-66.
33. Opherk C, Tronche F, Kellendonk C, et al. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol 2004; 18: 1346-53.
34. Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 2003; 72: 137-74.
35. Miura T, Ouchida R, Yoshikawa N, et al. Functional modulation of the glucocorticoid receptor and suppression of NF-kappaB-dependent transcription by ursodeoxycholic acid. J Biol Chem 2001; 276: 47371-8.
36. Jenniskens M, Langouche L, Vanwijngaerden YM, Mesotten D, Van den Berghe G. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med. 2016; 42: 16-27.
37. McMillin M, Frampton G, Quinn M, et al. Suppression of the HPA Axis During Cholestasis Can Be Attributed to Hypothalamic Bile Acid Signaling. Mol Endocrinol 2015; 29: 1720-30.
38. Krahulik D, Zapletalova J, Frysak Z, Vaverka M. Dysfunction of hypothalamic-hypophysial axis after traumatic brain injury in adults. J Neurosurg 2010; 113: 581-4.
39. Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet 1991; 337: 582-3.
40. Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288: 862-71.
41. Widmer IE, Puder JJ, König C, et al. Cortisol response in relation to the severity of stress and illness. J Clin Endocrinol Metab 2005; 90: 4579-86.
42. Loisa P, Uusaro A, Ruokonen E. A single adrenocorticotropic hormone stimulation test does not reveal adrenal insufficiency in septic shock. Anesth Analg 2005; 101: 1792-8.
43. Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008; 36: 1937-49.
44. Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med 2017; 43: 1751-63.
45. Venkatesh B, Finfer S, Cohen J, et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med 2018; 378: 797-808.
46. Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med 2018; 378: 809-18.
47. Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358: 111-24.
48. Rygård SL, Butler E, Granholm A, et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018; 44: 1003-16.
49. Rochwerg B, Oczkowski SJ, Siemieniuk RAC, et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit. Care Med. 2018; 46: 1411-20.
