RAÚL A. BORRACCI1, SILVIA V. SEGAL2, JOSÉ H. MÉNDEZ3
1Bioestadística, Facultad de Ciencias Biomédicas, Universidad Austral, 2Hospital de Niños Ricardo Gutiérrez, 3Departmento de Pediatría, Hospital Alemán, Buenos Aires, Argentina
Abstract Our objective was to develop and test a dynamic simulation model of human papillomavirus (HPV)- related diseases to assess rational vaccination strategies in Argentina. A dynamic stochastic transmission model for hetero- and homosexual transmission of HPV oncogenic and low-risk oncogenic types among females and males was developed. The model included HPV transmission and vaccination, the natural history of HPV-related diseases, disease outcomes, and cervical cancer screening. Considering all cervical cancers, covered or not by the current quadrivalent vaccine, the existing coverage rate would lead to 60% reduction in the global incidence of cervical cancer at 25 years, and to 79% at 50 years. Isolated current female vaccination without a screening program would need around 100 years to eliminate cervical cancer from the local population. Current coverage rate would lead to 59% reduction of vulvar cancer, 76% of vaginal cancer, 85% of anal cancer, and 87% of oropharyngeal cancer, estimated over a 25-year time prospect. Female HPV vaccination within the context of current cervical cancer screening should reach a minimum long-term mean coverage of 60% of girls, receiving at least a two-dose vaccine schedule, to significantly reduce or virtually eliminate cervical cancer at 50 years. Including vaccination to boys to improve herd immunity did not influence the incidence of cervical cancer over time, as long as female coverage did not fall below 50%. Regarding vulvar, vaginal, anal, penile, and some oropharyngeal cancers, current girls-only based vaccination could virtually eliminate these cancer types after 35-40 years, both in women and men.
Key words: human papillomavirus, vaccines, cancer, epidemiology, mathematical model
Resumen Modelo epidemiológico dinámico de enfermedades relacionadas al papilomavirus humano para evaluar estrategias de vacunación en la Argentina. Se desarrolló un modelo de simulación dinámica de enfermedades relacionadas con papilomavirus humano (VPH) para evaluar estrategias de vacunación. Se desarrolló un modelo dinámico estocástico para transmisión hetero/homosexual de VPH oncogénicos y de bajo riesgo oncogénico, entre mujeres y hombres. El modelo incluyó transmisión y vacunación contra VPH, historia natural de enfermedades relacionadas con VPH, mortalidad y programas de detección de cualquier cáncer de cuello uterino (CCU); teniendo en cuenta todos estos, con o sin vacunación cuadrivalente con la cobertura actual, la reducción sería 60% en la incidencia global de CCU en 25 años, y de 79% en 50 años. Vacunando solo mujeres, sin programa de detección precoz, necesitaría unos 100 años para eliminar el CCU localmente. La tasa de vacunación actual determinaría 59% de reducción del cáncer de vulva, 76% del cáncer vaginal, 85% del cáncer anal y 87% del cáncer orofaríngeo, a 25 años. La vacunación de mujeres, con el cribado actual del CCU, deberá alcanzar una cobertura media mínima a largo plazo del 60% de las niñas, con al menos dos dosis de vacunas, para reducir significativamente o eliminar el CCU en 50 años. La vacunación en niños para mejorar la inmunidad de grupo no influiría en la incidencia del CCU de n o caer la cobertura femenina por debajo de 50%. Con respecto a cánceres de vulva, vagina, ano, pene y algunos orofaríngeos, la vacunación actual solo en niñas podría eliminar virtualmente estos tipos de cáncer después de 35-40 años, tanto en mujeres como en hombres.
Palabras clave: papilomavirus humano, vacunas, cáncer, epidemiología, modelo matemático
Recibido: 6-VIII-2018 Aceptado: 3-IX-2018
Dirección postal: Dr. Raúl A. Borracci, La Pampa 3030, 1428 Buenos Aires, Argentina
e-mail: raborracci@gmail.com
A comprehensive approach to the evaluation of preventive strategies for human papillomavirus (HPV)-related diseases in developing countries raises a number of technical challenges. These include accurate modeling of HPV transmission within the local population, the natural history of HPV-associated cancers, screening test performance and coverage, population access to treatment, and vaccination uptake1. Since an age-specific population-based vaccination strategy –such as the school mandate for HPV vaccination in Argentina– is expected to reduce HPV-associated cancers only within two or three decades, accurate local projections are needed to support current and future financial investments. From the point of view of both public health and ethics, identification of the most effective strategies is essential when access to health services is limited2.
Although there are some general simulation models developed in high-income countries or supported by the pharmaceutical industry, country-specific data can affect the results of direct parametrization or calibration of progression and regression rates in local models. Nation-wide representative data, burden of HPV infection, screening coverage, urban/rural ratio population, and economic parameters could be the most changing factors between developed and developing countries. The first report from Argentina on a cost-effectiveness Markov model for HPV vaccination appeared in 2009 and it was rather linked to the industry that developed the HPV vaccine3. Other general cohort models involving Argentina4 estimated the perspective of vaccination in Latin American countries exclusively from an economical point of view5, 6. For modeling, a Markov cohort process of HPV-related diseases usually simulates a simple birth cohort study tracing people through their lives. Therefore, it can only estimate the direct effect on vaccinated groups, and not the changing effect of vaccination over time on unvaccinated persons. This indirect benefit of vaccination is referred to as “herd immunity”. That is why cohort models tend to underestimate the overall effectiveness of vaccination and herd immunity can be only captured with dynamic models in which HPV transmission is directly simulated in the whole population7, 8. Mathematically, dynamic modeling uses a set of differential equations to capture nonlinear interactions of disease transmission9. Basically, a dynamic simulation model assumes that the force of infection at time t is a function of the number of infectious individuals in the population at that time, and not a constant rate. Hence, mass immunization can reduce infectious individuals in the community and act on those who are not immunized10, 11. Conversely, because of their complexity, a weakness of the dynamic models is that they may appear as “black boxes” for decision makers.
HPV infection is also linked to other anogenital cancers (anus, vulva, vagina, and penis) and oropharyngeal cancers (tongue, tonsils, and oropharynx), whose incidence has increased in recent years in developed countries12, 13. Thus, it can be assumed that prophylactic vaccination would play a significant role in preventing these HPV-associated cancers, too. Until now, no general model evaluated vaccination strategies for these HPV-related diseases in Argentina, particularly using dynamic models to assess herd immunity impact.
The objective of this study was to develop and test a dynamic simulation model of HPV-related diseases to assess rational vaccination strategies in the Argentine population.
Materials and methods
The structure of a dynamic model is typically nonlinear, and differs from cohort models in that it does not track just a single cohort but rather the changing population over time. Hence, individuals constantly enter the model as they are born and exit as they die. In the model, vaccination reducing the prevalence of HPV infection over time means that susceptible individuals are less likely to become infected because there are fewer persons in the population to infect them with HPV. This indirect benefit is referred to as the herd immunity benefits of vaccination, and can be quantitatively evaluated only with dynamic models. In this study, we used the Stella software (v9.0.2, High Performance Systems, Hanover, NH) to develop dynamic stochastic transmission models for heterosexual and homosexual transmission of HPV oncogenic and low-risk oncogenic types 6/11/16/18/31/33/45/52 among females and males. The complete model included HPV transmission and vaccination, the natural history of HPV-related diseases, disease outcomes, and cervical cancer screening. Implementation was based on the schematic diagram of Figure 1. This model estimated the annual incidence of HPV-related precancerous lesions and invasive cervical cancer, as well as resulting cervical cancer deaths. In addition, other types of HPV-related male and female cancers were included in the model, such as vaginal, vulvar, anal, penile and oropharyngeal, as well as anogenital warts and recurrent respiratory papillomatosis (RRP). A steady-state solution was approached in the long run, until HPV-related cancers were virtually eliminated from the population. Model input data and assumptions, and Tables S1 to S3 are presented in the Appendix.
Because of the large number of equations and inputs, a sensitivity analysis was performed only on vaccination coverage since it was considered the most influential variable. The series of values obtained from sequential simulations were expressed as mean and standard deviation (SD), and comparisons were done with Student-t test assuming normal distribution. When a range of values was present, parameters were included in the model as random uniform probabilities between the minimum and maximum extremes of each parameter. Consequently, these stochastic simulations generated plots with visually saw wave time courses. For numerical analysis purposes, the Runge-Kutta 4th order method was used to solve the differential equations generated in the model. Although the equations’ solution may differ with the method used, no significant differences were found when using either Euler or 2nd and 4th order Runge-Kutta approaches.
Results
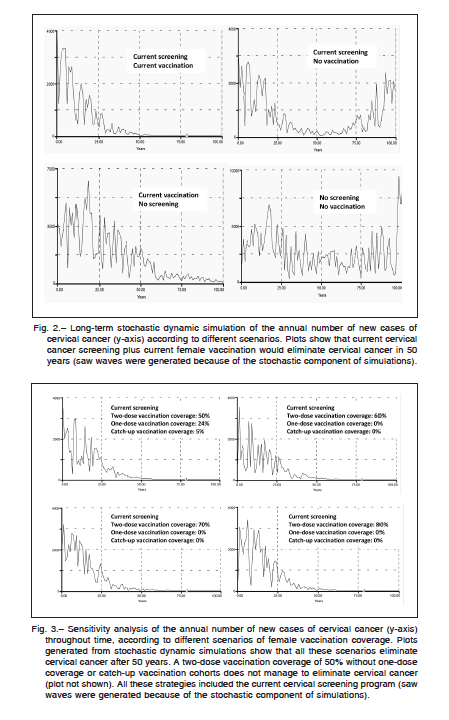
An example of long-term stochastic dynamic simulation of the annual number of new cases of cervical cancer according to different scenarios is shown in Figure 2. The results suggest that in Argentina, maintaining the current mean vaccination coverage rate of 70% for a two-dose application, 24% for one-dose application, and 5% of catch-up coverage, the vaccination strategy including only girls aged 11 years would be enough to virtually eliminate cervical cancer within 50 years, as long as the current cervical screening program persisted. Considering all cervical cancers, covered or not by the current quadrivalent vaccine, the existing coverage rate would lead to 60% (SD: 12.8%) reduction in the global incidence of cervical
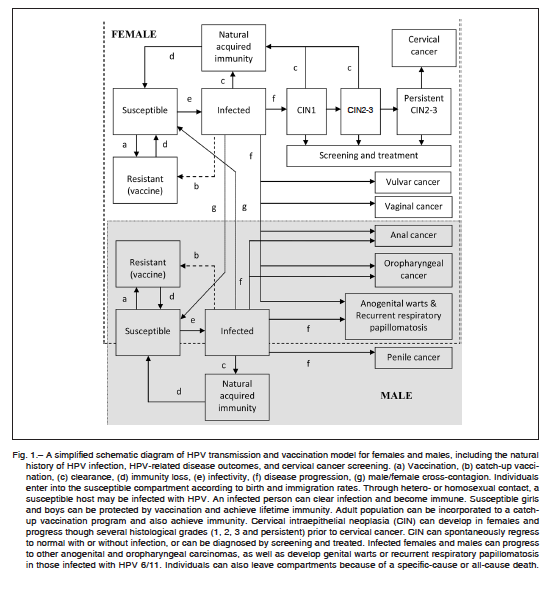
cancer at 25 years, and to 79% (SD: 9.9%) at 50 years. On the other hand, isolated current female vaccination without a screening program would need around 100 years to eliminate cervical cancer from the local population.
Figure 3 shows the annual number of new cases of cervical cancer throughout time, according to different scenarios of exclusive female vaccination coverage. Results from dynamic simulations suggested that all these scenarios would eliminate cervical cancer after 50 years, as long as the current cervical screening program remained constant. It should be noted that a two-dose vaccination coverage of 50% of the female population, without one-dose coverage or catch-up vaccination cohorts did not eliminate cervical cancer in the long term. In short, the sensitivity analysis demonstrates that a program covering over time at least 60% of girls aged 11 years with a two-dose coverage could significantly reduce cervical cancer in 25 years and virtually eliminate it in 50 years.
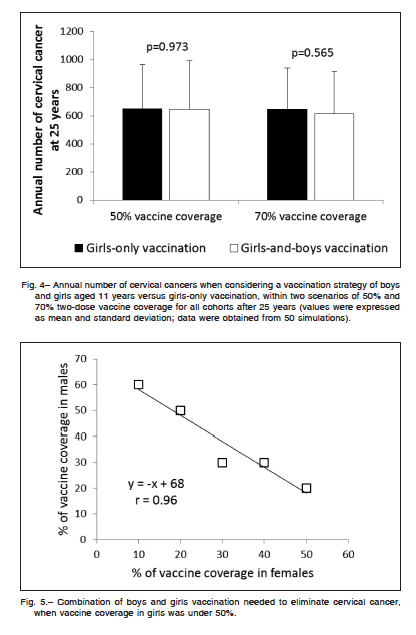
Extending vaccination to boys was proposed to enhance herd immunity and allows a further reduction of female HPV-related cancers. Figure 4 shows the annual number of cervical cancers when considering a vaccination strategy of boys and girls aged 11 years versus girls-only vaccination, within two scenarios of 50% and 70% two-dose vaccine coverage for all cohorts after 25 years. Simulations suggested that, independently of vaccine coverage, the inclusion of boys’ vaccination did not manage to reduce the annual number of cervical cancer
at 25 years. When vaccine coverage in girls was under 50%, the model suggested that the optimal combination of boys and girls vaccination required to eliminate cervical cancer follows a near negative linear relationship (Fig. 5). For instance, a vaccination rate in girls of only 40% would require vaccinating of at least 30% of boys to meet the goal of eliminating cervical cancer at 50 years.
The hypothetical influence of vaccination on vulvar, vaginal, anal, penile, and oropharyngeal cancers, both in women and men, is shown in Figure 6. Long-term simulations demonstrated that current female vaccination without male vaccination would virtually eliminate the fraction of these cancers associated to HPV in about 40 years in women, and 35 years in the male population. The images in the right column of the figure show that under this vaccination program, the long-term growth of these cancers is based on non HPV-related forms. Considering HPV- and non HPV-related cancers collectively, the cur-
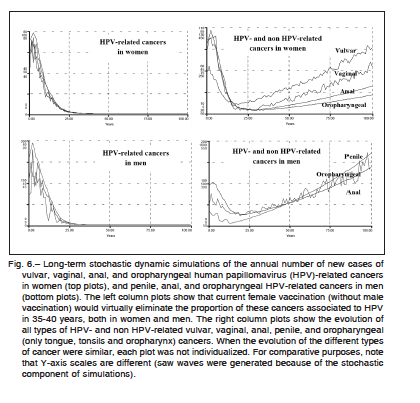
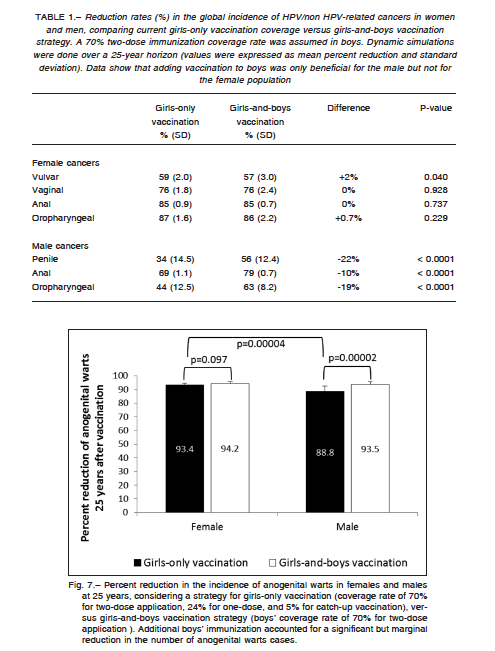
rent coverage rate would lead to 59% (SD: 2.0%) reduction in the global incidence of vulvar cancer, 76% (SD: 1.8%) for vaginal cancer, 85% (SD: 0.9%) for female versus 70% (SD: 1.1%) for male anal cancer (p < 0.0001), 34% (SD: 14.5%) for penile cancer, and 87% (SD: 1.6%) for female versus 44% (SD: 12.5%) for male oropharyngeal cancer (p < 0.0001), all reductions estimated over a 25- year time prospect. Extending the vaccination program to boys significantly changed the cancer reduction rates in males but not in women, probably due to the effect of the fraction of males who have sex with males (Table 1).
Figure 7 shows the percent reduction in the incidence of anogenital warts in females and males at a 25-year simulation, according to girls-only and boys-and-girls vaccination strategies. The current girls-only vaccination program would lead to a higher reduction of anogenital warts in females than in males; nevertheless, males could achieve a similar reduction by adding a 70% two-dose immunization coverage rate in boys. Finally, RRP occurring in children below 11 years and in the adult population could be virtually eliminated at 25 years with the current girls-only immunization program. No benefit was observed when boys’ vaccination was added to the model.
Discussion
Cervical cancer peaks in women aged 45 years and above; consequently, most of population health gains for cervical cancer prevention are likely to be observed several decades after HPV vaccination implementation. Therefore, mathematical models play a key role in evaluating long-term predictions of health benefits for policy decision-making, especially in developing countries with limited resources. In most countries, HPV vaccination rates are low compared with rates for other recommended vaccines. In Argentina, vaccine coverage increased from negligible levels before 2011 to a national average of 87.9% for the first dose, 71.6% for the second dose, and 52.2% for the third dose in 2013; nevertheless, there is a large variance in coverage across provinces17, 51. In the present study, we developed an epidemiological dynamic model able to explore HPV vaccination strategies within the local context. Globally, the present long-term stochastic simulation revealed that maintaining a mean two-dose vaccine coverage of ≥60% only for girls aged 11, cervical cancer could be drastically reduced or virtually eliminated in a 50-year term, as long as the current screening rate were maintained.
Recent evidence-based guidelines of the American Society of Clinical Oncology recommended two doses of HPV vaccine for girls aged 9 to 14 years, and if more than 50% coverage in the female population were achieved, then boys could be vaccinated to prevent other non-cervical HPV-related cancers and diseases52. Furthermore, a third dose of HPV vaccine is unlikely to be cost-effective if duration of two-dose protection is longer than 30 years53.
Updated studies suggest that vaccination of pre-adolescent males may be cost-effective if vaccination coverage in females cannot be increased above ~50%. However, the present analysis found that, assuming overall vaccination coverage of 50% or 70%, the vaccination of both girls and boys was not associated with reduction of cervical cancer versus a strategy of girls-only vaccination. Furthermore, these findings are in coincidence with other modeling studies, which concluded that the incremental impact of vaccinating boys was limited54-57. Only the study by Marty el al.57 indicated that vaccination of both girls and boys was associated with notable incremental clinical benefits; however, this last study may be partially biased by the authors’ competing interests.
The present study also recognized the value of older female catch-up vaccination for two-dose borderline coverage. Likewise, a recent research based on a Markov model concluded that extending vaccination to older girls and females (catch-up vaccination), instead of adding boys’ immunization would maximize the number of cervical cancer cases prevented58.
The scope of the present study was not limited to cervical cancer but also accounted for other HPV-related neoplasms. Although those other cancers are less commonly related to HPV than cervical carcinomas, vulvar, vaginal, penile, anal, and oropharyngeal types were drastically reduced with the current girls-only vaccination coverage, as simulated over a 35-40-year time horizon. Extra vaccination on the boys’ cohort modified reduction rates of these cancers in men but not in women.
A model developed by Elbasha et al.59 showed that by vaccinating girls alone, an 83% reduction in the incidence of anogenital warts was expected, but this reduction increased to 97% if boys were also vaccinated. A random network model assuming a vaccine coverage of 73% in girls-only and catch-up coverage rates decreasing with age to 52% for 20-26 year-olds, calculated a 59% reduction in anogenital wart cases47. Another research concluded that focusing on attaining high HPV vaccination coverage of girls, rather than including boys in a vaccination program, may be a more efficient strategy to reduce anogenital warts in low-resource settings60. The current model showed a drastic reduction in the incidence of anogenital warts both in females and males at 25 years, by vaccinating girls alone; it also indicated that an extra vaccination in boys would provide a significant but marginal reduction of nearly 5% in the male incidence of warts.
Recurrent respiratory papillomatosis is a condition characterized by the repeated growth of benign exophytic papillomata in the larynx. In the juvenile-onset form of this disease, perinatal transmission of HPV is thought to occur intrapartum from infected mother to child, and must be differentiated from the adult form49. The current model ran two separate simulations for children and adult cohorts. At 25 years, both simulations showed virtual disappearance of RRP with the current girls-only vaccination strategy, and no benefits were observed when incorporating immunization to boys.
Future modeling applications should explore how cervical cancer screening strategies might evolve over time following the onset of HPV vaccination in Argentina, particularly for underserved women. In this research line, the present study suggests the possibility of virtually eliminating cervical cancer with a rational vaccination strategy after a 100-year period, even in the absence of any type of cervical cancer screening program. However, since about 18% of cervical cancer would not be covered by the current quadrivalent vaccine, cervical screening programs should remain as the best known prevention practice for early diagnosis and treatment.
Even though HPV vaccine seems to have been firmly established, the evaluation of HPV vaccination programs around the world is still an active research area61-66. It has been recognized that once a national vaccination program is initiated, it is difficult to influence the design and impose a monitoring mechanism to gather information1. However, and specially for developing countries, to construct a pilot model of vaccination coverage could be used to improve the understanding of the dynamic effects of vaccination and to recalculate regional long-term health goals. Other classical dynamic models have been published to date67-70. Two models focused on HPV infection only, and not on subsequent HPV diseases66, 69. Barnabas et al.69 modeled the potential epidemiologic impact of an HPV vaccine in an unscreened developing-world population; Taira et al.68 combined a cohort model with a transmission model into an hybrid system.
This study has some limitations. First, these dynamic models explored only HPV-related cancer; other etiological presentations of vulvar, vaginal, anal, penile and oropharyngeal carcinomas were marginally modeled and included in the outcomes. Second, all simulations were done considering a homogeneous mean coverage vaccination rate over time throughout the whole country. Therefore, models did not consider the fact that there are local populations where both women and men are protected because of the high vaccination coverage rate; whereas in populations with low vaccination coverage that would potentially benefit from vaccination of males (herd protection), the vaccine does not reach either group. Additional regional simulations should be performed to compensate for local heterogeneities. Third, the model did not offer a cost-effectiveness evaluation; nevertheless, if there were reliable costs, they could be easily incorporated into the model in future research. Fourth, cohorts were not divided into age-specific or sexual activity class reflecting a different risk for acquiring the infection; conversely, the population was analyzed as a whole. Some recent research in Argentina found a low degree of knowledge about HPV infection and its prevention in the population71, and a low rate of vaccination acceptance in other selected communities72. These key points should be included in future models. Much of the current consensus on how anal dysplasia evolves is derived directly from cervical cancer evolution12; nevertheless, as there is no universal accepted algorithm for anal cancer screening, early detection programs were not included in the model. Finally, all the outcomes should be considered in the context of widely accepted rational assumptions, and based on the accuracy of the model parameter set obtained from the literature.
In conclusion, this study intends to be a translational academic paper for health policy application. A mathematical dynamic model focusing on both the infection and the disease process was developed to explore the population-level impact of the Argentine HPV vaccination program on the incidence of HPV-related cancers. This model analyzed the effect of different levels of vaccination coverage, both in females and males, to propose rational epidemiological and policy-making strategies to reduce HPV-related diseases in Argentina in the long term. Female HPV vaccination within the context of current cervical cancer screening should reach a minimum long-term mean coverage of 60% of girls aged 11 years, receiving at least a two-dose vaccine schedule, to significantly reduce or virtually eliminate cervical cancer at 50 years. Different combinations of one- and two-dose protection plus catch-up vaccination programs in older females also showed to be beneficial options. Including vaccination to boys aged 11 to improve herd immunity did not influence the incidence of cervical cancer over time, as long as the female coverage did not fall below 50%. Regarding vulvar, vaginal, anal, penile, and some oropharyngeal cancers, current girls-only based vaccination could virtually eliminate these cancer types after 35-40 years, both in women and men, with extra benefits only in males when adding simultaneously boys’ vaccination. A similar outcome is expected for anogenital warts and RRP at a 25 year horizon, but the benefit for males when adding boys’ immunization was marginal for warts and nil for RRP. Results from the current model can be instrumental for evaluating HPV vaccination policies in Argentina.
Acknowledgments: The authors thank Florencia Nolte, Angela Gentile and Norberto Giglio from the Division of Health Promotion and Protection, Hospital de Niños Ricardo Gutiérrez, Buenos Aires, for critical review of the manuscript.
Conflict of interests: None to declare
References
1. Craig BM, Brisson M, Chesson H, Giuliano AR, Jit M, Moffitt HL. Proceedings of the Modeling Evidence in HPV Pre-Conference Workshop in Malmö, Sweden, May 9-10, 2009. Clin Ther 2010; 32: 1546-64.
2. Natunen K, Lehtinen J, Namujju P, Sellors J, Lehtinen M. Aspects of prophylactic vaccination against cervical cancer and other human papillomavirus-related cancers in developing countries. Infect Dis Obstet Gynecol 2011; 2011: 675858.
3. Colantonio L, Gómez JA, Demarteau N, Standaert B, Pichón Riviére A, Augustovski F. Cost-effectiveness analysis of a cervical cancer vaccine in five Latin American countries. Vaccine 2009; 27: 5519-29.
4. Pichon-Riviere A, Alcaraz A, Caporale J, et al. Costo-efectividad de la vacuna tetravalente contra VPH en Argentina, a partir de un modelo dinámico de transmisión. Salud Publica Mex 2015; 57: 504-13.
5. Goldie SJ, Diaz M, Constenla D, Alvis N, Andrus JK, Kinm SY. Mathematical models of cervical cancer prevention in Latin America and the Caribbean. Vaccine 2008; 26 (Suppl 11): L59-72.
6. Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health 2014; 2: e406-14.
7. Canfell K, Chesson H, Kulasingam SL, Berkhof J, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine 2012; 30 (Suppl 5): F157-67.
8. Dasbach EJ, Elbasha EH, Insinga RP. Mathematical models for predicting the epidemiologic and economic impact of vaccination against human papillomavirus infection and disease. Epidemiol Rev 2006; 28: 88-100.
9. Boily MC, Mâsse B. Mathematical models of disease transmission: a precious tool for the study of sexually transmitted diseases. Can J Public Health 1997; 88: 255-65.
10. Edmunds WJ, Medley GF, Nokes DJ. Evaluating the cost effectiveness of vaccination programmes: a dynamic perspective. Stat Med 1999; 18: 3263-82.
11. Brisson M, Edmunds WJ. Economic evaluation of vaccination programs: the impact of herd immunity. Med Decis Making 2003; 1: 76-82.
12. Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: a review. World J Gastrointest Surg 2016; 8: 41-51.
13. Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related epidemic – A review of epidemiology, biology, virus detection and issues in management. Lancet Oncol 2010; 11: 781-9.
14. Censo nacional de población, hogares y viviendas 2010: censo del Bicentenario: resultados definitivos, Serie B n° 2. – 1a ed. – Buenos Aires: Instituto Nacional de Estadística y Censos – INDEC, 2012.
15. Basic indicators, Argentina. Ministerio de Salud de la Nación. Pan American Health Organization – PAHO/ WHO, 2016. En: http://iris.paho.org/xmlui/handle/ 123456789/34028; consultado abril 2017.
16. Bruni L, Barrionuevo-Rosas L, Albero G, et al. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Argentina. Summary Report 15 December 2016. En: http://www.hpvcentre.net/statistics/ reports/ARG.pdf; consultado abril 2017.
17. Katz N, Gaiano A, Pérez Carrega M, Vizzotti C. Vacuna contra el virus del papiloma humano: resultados a un año de su incorporación al calendario nacional de vacunación. Rev Argent Salud Pública 2013; 4: 44-6.
18. Goldhaber-Fiebert JD, Stout NK, Ortendahl J, Kuntz KM, Goldie SJ, Salomon JA. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul Health Metr 2007; 5: 11.
19. Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 2003; 88: 63-73.
20. Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121: 621-32.
21. Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst 1999, 91: 252-8.
22. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998, 92: 727-35.
23. Demarteau N, Morhason-Bello IO, Akinwunmi B, Adewole IF. Modeling optimal cervical cancer prevention strategies in Nigeria. BMC Cancer 2014; 14: 365.
24. Lee SL, Tameru AM. A mathematical model of human papillomavirus (HPV) in the United States and its impact on cervical cancer. J Cancer 2012; 3: 262-8.
25. Barnabas RV, Laukkanen P, Koskela P, Kontula O, Lehtinen M, Garnett GP. Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modeling analyses. PLoS Medicine 2006; 3: e138.
26. PAHO/WHO Mortality Database, 2012. En: http://www. paho.org/hq/index.php?option= com_docman&task=doc_ view&gid=22979&Itemid=270&lang=en; consultado abril 2017.
27. Wu M, Li J, Lu H, Wang L, Zhang B, Lin Z. Impact of the care provided by gynecologic oncologists on outcomes of cervical cancer patients treated with radical hysterectomy. Onco Targets Ther 2016; 9: 1361-70.
28. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131: 2349-59.
29. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893-917.
30. Arrossi S, Ramos S, Paolino M, Sankaranarayanan R. Social inequality in Pap smear coverage: identifying under-users of cervical cancer screening in Argentina. Reprod Health Matters 2008; 16: 50-8.
31. de Sanjosé S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 2013; 49: 3450-61.
32. Polterauer S, Schwameis R, Grimm C, et al. Prognostic value of lymph node ratio and number of positive inguinal nodes in patients with vulvar cancer. Gynecol Oncol 2017; 147: 92-7.
33. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13: 607-15.
34. De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer 2009; 124: 1626-36.
35. Alemany L, Saunier M, Tinoco L, et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer 2014; 50: 2846-54.
36. Yagi A, Ueda Y, Kakuda M, et al. Descriptive epidemiological study of vaginal cancer using data from the Osaka Japan population-based cancer registry: Long-term analysis from a clinical viewpoint. Medicine (Baltimore) 2017; 96: e7751.
37. Walker AK, Kartsonaki C, Collantes E, Nicholson J, Gilbert DC, Kiltie AE. No additional prognostic value for MRE11 in squamous cell carcinomas of the anus treated with chemo-radiotherapy. Br J Cancer 2017; 117: 322-5.
38. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males. Advisory Committee on Immunization Practice (ACIP). 2011. MMWR Morb Mortal Wkly Rep 2011; 60: 1705-8.
39. Cáceres C, Konda K, Pecheny M, Chatterjee A, Lyerla R. Estimating the number of men who have sex with men in low and middle income countries. Sex Transm Infect 2006; 82 (Suppl 3): iii3-iii9.
40. Picconi MA, Eiján AM, Distéfano AL, et al. Human papillomavirus (HPV) DNA in penile carcinomas in Argentina: analysis of primary tumors and lymph nodes. J Med Virol 2000; 61: 65-9.
41. Arya M, Li R, Pegler K, et al. Long-term trends in incidence, survival and mortality of primary penile cancer in England. Cancer Causes Control 2013; 24: 2169-76.
42. Gonzalez J, Gutierrez R, Keszler A, et al. Human papillomavirus in oral lesions. Medicina (B Aires) 2007; 67: 363-8.
43. Ribeiro KB, Levi JE, Pawlita M, et al. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol 2011; 40: 489-502.
44. Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/ E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 2014; 15: 1319-31.
45. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14: 467-75.
46. Hall SF, Liu FF, O’Sullivan B, et al. Did the addition of concurrent chemotherapy to conventional radiotherapy improve survival for patients with HPV+ve and HPV-ve oropharynx cancer? A population-based study. Br J Cancer 2017; 117: 1105-12.
47. Díez-Domingo J, Sánchez-Alonso V, Villanueva RJ, Acedo L, Moraño JA, Villanueva-Oller J. Random network models to predict the long-term impact of HPV vaccination on genital warts. Viruses 2017; 9: 300.
48. Seedat RY, Schall R. Age of diagnosis, incidence and prevalence of recurrent respiratory papillomatosis- A South African perspective. Clin Otolaryngol 2018; 4: 533-7.
49. Novakovic D, Cheng ATL, Zurynski Y, et al. A prospective study of the incidence of juvenile-onset recurrent respiratory papillomatosis after implementation of a National HPV Vaccination Program. J Infect Dis 2018; 217: 208-12.
50. Eftekhaar NS, Niya MHK, Izadi F, Teaghinezhad-S S, Keyvani H. Human papillomavirus (HPV) genotype distribution in patients with recurrent respiratory papillomatosis (RRP) in Iran. Asian Pac J Cancer Prev 2017; 18: 1973-6.
51. Patel H, Wilson E, Vizzotti C, Parston G, Prestt J, Darzi A. Argentina’s successful implementation of a National Human Papillomavirus Vaccination Program. Health Aff (Millwood) 2016; 35: 301-8.
52. Arrossi S, Temin S, Garland S, et al. Primary prevention of cervical cancer: American Society of Clinical Oncology Resource-Stratified Guideline. J Glob Oncol 2017 ; 3: 611-34.
53. Laprise JF, Drolet M, Boily MC, et al. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modeling study. Vaccine 2014; 32: 5845-53.
54. Smith MA, Lew JB, Walker RJ, Brotherton JM, Nickson C, Canfell K. The predicted impact of HPV vaccination on male infections and male HPV related cancers in Australia. Vaccine 2011; 29: 9112-22.
55. Brisson M, van de Velde N, Franco EL, Drolet M, Boily MC. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J Infect Dis 2011; 204: 372-6.
56. Bogaards JA, Kretzschmar M, Xiridou M, Meijer CJ, Berkhof J, Wallinga J. Sex specific immunization for sexually transmitted infections such as human papillomavirus: insights from mathematical models. PLoS Med 2011; 8: e1001147.
57. Marty R, Roze S, Bresse X, Largeron N, Smith-Palmer J. Estimating the clinical benefits of vaccinating boys and girls against HPV-related diseases in Europe. BMC Cancer 2013; 13: 10.
58. Bonanni P, Gabutti G, Demarteau N, Boccalini S, La Torre G. Vaccination of boys or catch-up of girls above 11 years of age with the HPV-16/18 HSO4-adjuvented vaccine: where is the greatest benefit for cervical cancer prevention in Italy? BMC Infect Dis 2015; 15: 377.
59. Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 2007; 13: 28-41.
60. Sharma M, Sy S, Kim JJ. The value of male human papillomavirus vaccination in preventing cervical cancer and genital warts in allow-resource setting. BJOG 2016; 123: 917-26.
61. Damm O, Horn J, Mikolajczyk RT, et al. Cost-effectiveness of human papillomavirus vaccination in Germany. Cost Eff Resour Alloc 2017; 15: 18.
62. Kosen S, Andrijono A, Ocviyanti D, Indriatmi W. The cost-effectiveness of quadrivalent human papillomavirus vaccination in Indonesia. Asian Pac J Cancer Prev 2017; 18: 2011-7.
63. Uhart M, Adam M, Dahlab A, Bresse X. Loss of chance associated with sub-optimal HPV vaccination coverage rate in France. Papillomavirus Res 2017 ; 3 : 73-9.
64. Mennini FS, Bonanni P, Bianic F, et al. Cost-effectiveness analysis of the nine-valent HPV vaccine in Italy. Cost Eff Resour Alloc 2017; 15: 11.
65. Tay SK, Hsu TY, Shcheprov A, Walia A, Kulkarni AS. The clinical and economic benefits of school-based quadrivalent HPV vaccination in Singapore. Int J Gynaecol Obstet 2017; 137: 129-37.
66. Laprise JF, Markowitz LE, Chesson HW, Drolet M, Brisson M. Comparison of 2-dose and 3-dose 9-valent human papillomavirus vaccine schedules in the United States: a cost-effectiveness analysis. J Infect Dis 2016; 214: 685-8.
67. Hughes JP, Garnett GP, Koutsky L. The theoretical population level impact of a prophylactic human papilloma virus vaccine. Epidemiology 2002; 13: 631-9.
68. Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004; 10: 1915-23.
69. Barnabas RV, Garnett GP. The potential public health impact of vaccines against human papillomavirus. In: Prendiville W, Davies P. The clinical handbook of human papillomavirus. Lancaster, United Kingdom: Parthenon Publishing/Parthenon Medical Communications, 2004.
70. Elbasha EH, Galvani AP. Vaccination against multiple HPV types. Math Biosci 2005; 197: 88-117.
71. Venezuela RF, Monetti MS, Kiguen AX, Frutos MC, Mosmann JP, Cuffini CG. Knowledge of the general community in Cordoba, Argentina, on human papilloma virus infection and its prevention. Asian Pac J Cancer Prev 2016; 17: 2689-94.
72. Chaparro RM, Em Vargas V, Zorzo LR, Genero S, Cayre A. Acceptance of human papillomavirus vaccination and associated factors in the city of Resistencia, Argentina. Arch Argent Pediatr 2016; 114: 36-43.
Appendix
Model input data
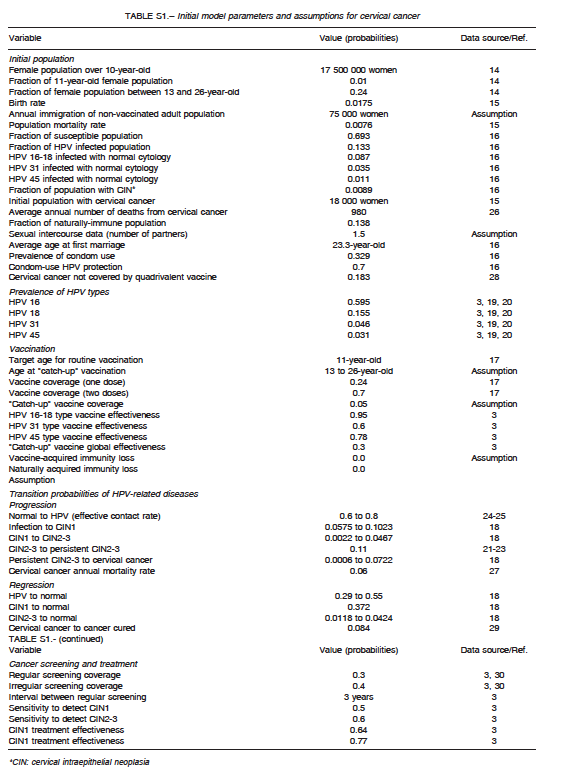
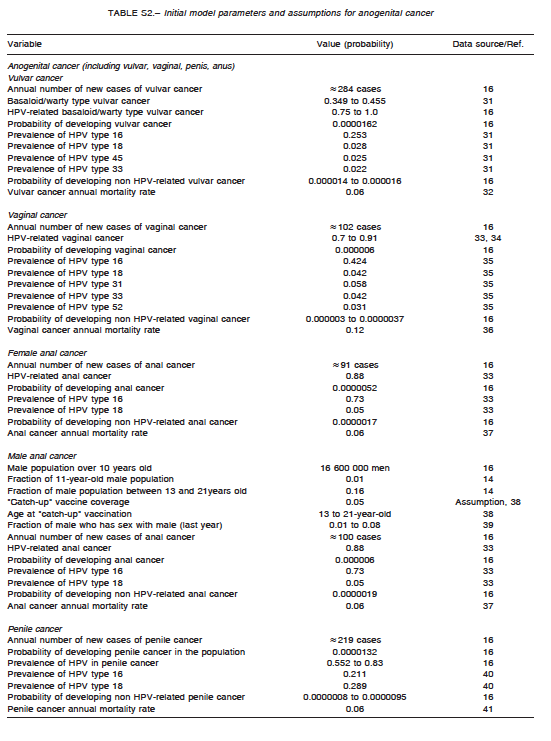
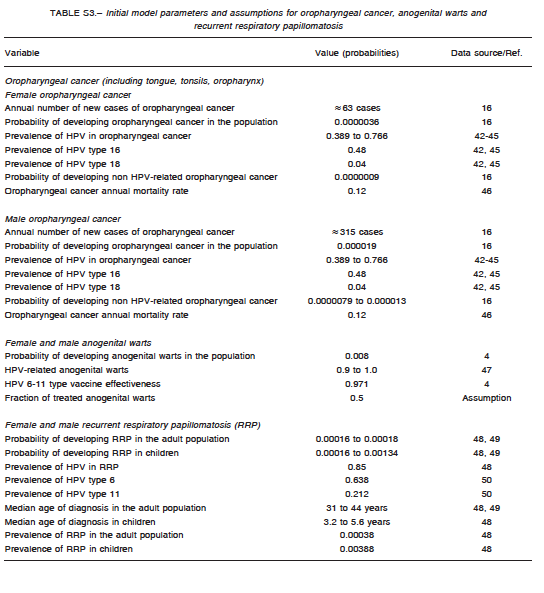
A comprehensive search of the literature was conducted to obtain baseline values for model parametrization. They are summarized in Table S1 for cervical cancer, Table S2 for anogenital cancer, and Table S3 for oropharyngeal cancer. The analysis incorporated female-specific conditions including HPV-related cervical, vulvar and vaginal carcinomas, penile carcinoma in males, and anal carcinoma, genital warts, recurrent respiratory papillomatosis (RRP), and head and neck cancers in both males and females. Epidemiologic input data concerning the incidence of HPV-related diseases in Argentina were derived from previously published epidemiologic studies. Regarding cervical cancer, the model incorporated both HPV vaccination and screening programs. Specific progression rate of HPV-16 and HPV-18 to different stages of cervical dysplasia and cancer were estimated from the literature. Current estimates regarding Pap screening, lesion treatment, cancer progression and mortality were also considered. Sexual behavior was introduced in the model by including average age at first marriage, mean number of partners, condom use rate, and the fraction of males who have sex with males. Finally, the impact of non-vaccinated adult people migrating to the country was taken into account by adding an annual fixed number of infected and non-infected migrants to the model. Calibration for all types of cancers was done to adjust the model outcomes to their annual incidences reported for Argentina. To validate the model, the incidence of cancer cases and deaths predicted by the model was compared with those reported by the National Ministry of Health1-16. The annual rates of cancer cases of the current model matched accurately local data after calibration.
Assumptions
For modeling purposes, the following assumptions were considered: HPV is carried and sexually transmitted by both females and males; routine HPV vaccination is considered to be effective in pre-adolescent or early adolescent girls or boys prior to sexual debut (HPV DNA negative); catch-up vaccination cohorts are more likely to have prior exposure to HPV infection; and preventable HPV-related cancers in males are considerably lower than those in females, but vaccination of males can potentially affect transmission to females and other males. The vaccine (both for girls-only and boys-and-girls vaccination programs) was assumed to be administered to 11-year olds. Initially, a vaccine coverage of 70% was assumed for girls receiving at least two vaccination doses, and a vaccine coverage of 24% for those receiving one vaccination dose. One-dose coverage was taken into account as an imperfect adherence to the scheduled vaccination course, and decreased vaccine efficacy was also assumed for those having received one dose in comparison with those who were fully vaccinated (two doses).These figures represent the data for Argentina reported in 201317.
A four-year transition period was assumed from vaccination to first sexual contact, and the duration of vaccine protection was considered to be that of the patient’s lifetime. Finally, low-risk HPV types 6 and 11 were assumed to account for nearly all cases of anogenital warts and RRP.