OSCAR D. SALOMÓN 1, 2, ADRIANA A. PÉREZ 3, ADELINA R. RIARTE 4, NATALIA CASAS 5, VICTORIA FRAGUEIRO-FRÍAS 4, VANESA NEGRI 4, MARÍA SOLEDAD SANTINI 6, 2, DOMINGO J. LIOTTA 1, 7
1 Instituto Nacional de Medicina Tropical, ANLIS Carlos G. Malbrán, 2 Consejo Nacional de Investigaciones Científicas y Técnicas, 3 Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, 4 Instituto Nacional de Parasitología Dr. Mario Fatala Chaben, ANLIS CG Malbrán, 5 Coordinación de Zoonosis, Ministerio de Salud y Desarrollo Social de la Nación, 6 Centro Nacional de Diagnóstico e Investigación en Endemo-Epidemias, ANLIS Carlos G. Malbrán, Ministerio de Salud y Desarrollo Social de la Nación, 7 Laboratorio de Biología Molecular Aplicada, Facultad de Ciencias Exactas, Químicas y Naturales, Universidad Nacional de Misiones, Argentina
Resumen Para diagnosticar perros infectados por Leishmania infantum, en las Américas se utiliza ampliamente la prueba rápida rK39, mientras que DPP fue adoptado recientemente por Brasil. En este estudio se evaluó el desempeño de las pruebas rK39-RDT y DPP en escenarios de transmisión urbana reciente en Argentina. La sensibilidad y especificidad se evaluaron con un panel de sueros y muestras de campo, considerando muestras infectadas verdaderas aquellas con pruebas parasitológicas y/o de PCR positivas. Como ninguna de estas pruebas puede considerarse estándar de oro, el desempeño también se evaluó mediante análisis de clases latentes, una técnica de modelado estadístico que permite estimar sensibilidad y especificidad definiendo una variable de clase latente como estándar. La sensibilidad de ambas pruebas en el panel fue de alrededor del 92% (perros sintomáticos 96%, asintomáticos 83%), mientras que la sensibilidad en muestras de campo fue rK39-RDT: 77%, y DPP 98% (media en perros sintomáticos 89%, asintomáticos 82%). La especificidad fue similar para ambas pruebas y muestras, cerca de 98%. Por lo tanto, estas pruebas son aceptables para estudios programáticos caninos de base-poblacional, como estratificación espacial, intervención de foco y seguimiento, y podrían utilizarse para el tamizaje individual y la confirmación del diagnóstico clínico presuntivo en perros poli-sintomáticos. La incapacidad de discriminar entre inmunidad e infectividad real sugiere que se requerirá una combinación con otras pruebas, de base no inmunológica, para un diagnóstico suficientemente sensible/específico que permita definir las medidas de control en reservorios individuales, tanto para salud pública, como para la gestión individual en salud animal.
Palabras clave: diagnóstico, prueba inmunológica, vigilancia poblacional, leishmaniasis canina
Abstract To diagnose dogs infected by Leishmania infantum rK39 rapid diagnosis test is widely used in the Americas, while dual path platform (DPP) was recently adopted by Brazil. In this study we assessed the performance of rK39-RDT and DPP tests in recent urban transmission scenarios of Argentina. The sensitivity and specificity were evaluated with a sera panel and field samples, taken as true infected those from parasitological and/or PCR positive tests. Since none of these tests can be taken as a gold standard, the performance was also evaluated using Latent Class Analysis, a statistical modeling technique which allows to estimating sensitivity and specificity defining a latent class variable as the reference standard. The sensitivity of both tests in the panel was around 92% (symptomatic dogs 96%, asymptomatic 83%), while the sensitivity in field samples of rK39-RDT was 77%, and DPP 98% (mean in symptomatic dogs 89%, asymptomatic 82%). The specificity was similar for both tests and samples, around 98%. Therefore, these tests are acceptable for program dog population-based studies, as spatial stratification, focus intervention and follow up, and they could be used for individual screening and confirmation of clinical presumptive diagnosis in polysymptomatic dogs. The inability to discriminate between immunity and actual infectiousness suggest that a combination with other non-immunological based tests will be required for highly sensitive/specific diagnosis in order to targeting control measures in individual reservoirs from public health perspective, as for individual management from animal health perspective.
Key words: diagnosis, immunologic test, population surveillance, canine leishmaniasis
Postal address: Oscar D. Salomón, Instituto Nacional de Medicina Tropical, Almafuerte y Ámbar s/n, 3370 Puerto Iguazú, Misiones, Argentina
e-mail: odanielsalomon@gmail.com
Visceral leishmaniasis is a neglected vector-borne disease with an estimated worldwide annual incidence of 200 000 to 400 000 cases, and a case-fatality rate of 10% 1. Leishmania infantum is the etiological agent of the disease in the Americas, with Lutozmyia longipalpis as its main vector. The cases of Brazil account for 96% of the human visceral cases of the Americas, but an increasing incidence and expansion of transmission in Argentina, Colombia, Paraguay and Venezuela were reported during the last decades 2. In Argentina the urban vector was recorded for the first time in 2004, the first human case in 2006, and after that the vector spread to five provinces, and human cases to four provinces. This trend is due to the process of urbanization and expansion that started in Brazil during 1970-1980 3. The dog, despite its clinical status (asymptomatic to polysymptomatic), is the main urban reservoir of parasites transmitted both to dogs and humans through the bite of an infected vector. But there is also a dog to dog reservoir by dog vertical and sexual transmission. Therefore, the accurate diagnosis of canine visceral leishmaniasis (CVL) became a critical ‘One Health’ issue for human public health, and for individual and collective animal health.
Many immuno-serological and molecular diagnostic tests have been proposed and even are commercially available 4. However, in order to assess its performance, the aim of any new test should be defined: a) for control programs: population-based surveillance screening of reservoirs, cohort studies and impact assessment, individual-based management decision, identification of super-spreaders; b) for the veterinarian perspective and research: diagnosis for case management, biomarkers of disease prognosis, molecules for vaccine development and evaluation of effectiveness of treatment.
Since December 2011, the Brazilian Ministry of Health, requires for CVL immunoserodiagnosis, in the Surveillance and Control Program of Leishmaniasis, the dual-path platform DPP® rapid test for screening, with an enzyme-linked immunosorbent assay (ELISA) using soluble antigens of L. infantum as confirmatory test 5. Argentina Leishmaniasis’ Program use Kalazar DetectTM for CVL diagnosis, a rK39 dipstick rapid test commercially available with a recombinant protein of L.infantum, which is also contained in the modified rK28 DPP test.
Both rapid tests were compared in Brazil showing that the results could depend on the transmission scenario intensity 6.
Therefore, we performed a study to assess the performance of both tests in the relatively new foci of Argentina, in the southern latitudes of CVL spread, with a controlled sera panel and with field sampled dogs from an endemic city, so with a broad spectrum of immune responses to infection. The results can be useful to compare the Brazilian studies with those of other countries, mainly in border areas, to evaluate the usefulness of the DPP outside Brazil, but also to analyze the advantages and limitations of an immune-serological rapid tests for CVL diagnosis.
Materials and methods
Serological Qualitative Rapid Diagnostic Tests: a) rK39-RDT: Kalazar DetectTM (InBios, Inc., Seattle, WA, USA) kit for CVL.
It is based on a 39 amino acid repeat immunodominant Bcell epitope in a kinesin-related protein, which is conserved between L. infantum and L. donovani. b) DPP: Dual-path platform fast test (TR DPP®kit for CVL (Bio-Manguinhos, Rio de Janeiro, Brazil). It is a colloidal gold-based immunochromatography assay with a recombinant chimeric protein (rK28) multi-epitope from the fusion of L. infantum genes: k9, single repeat units of k39 and k26 7.
Parasitological test: Samples were taken by a popliteal lymph node puncture with a hypodermic needle; smears were fixed with methanol and stained with May-Grünwald-Giemsa.
Routine analysis involved the observation of two hundred microscopical fields, or until the identification of at least one amastigote.
PCR tests: a) Sample genome quality was evaluated with protocol targets the CytB gene of vertebrates (CytB1 5´-CCC CTC AGA ATG ATA TTT GTC CTC A-3´ and CytB2 5´-CCA TCC AAC ATC TCA GCA TGA TGA AA-3´). Human genome of a healthy donor was employed as positive control. b) Leishmania was detected using a standardized protocol targeting the ribosomal internal transcribed spacer (ITS-1) of Leishmania sp. (LITSR 5´-CTG GAT CAT TTT CCG ATG-3´ and L5.8S 5´-TGA TAC CAC TTA TCG CAC TT-3´)8. Leishmania (V.) braziliensis reference strain (MHOM/BR/1975/2903) was employed as positive control. PCRs were carried out with 5 μl of extracted DNA in a final volume of 50 μl containing 1× PCR buffer (200mM Tris-HCl, pH 8), 0.1mM EDTA, 1mM DTT, 50% glycerol (v/v) (Invitrogen™), 2 mM MgCl2 (Invitrogen™), 2.5% DMSO (SIGMA™), 0.2 mM dNTP Mix, 0.5 μM of each primer, and 1.4 U Taq polymerase (Invitrogen™). Up to 10 μl of the amplified products were analyzed by 2% agarose gel electrophoresis at 5 V/cm, stained with SYBR Safe™ (0.5 μg/ml) and visualized with a Safe Imager™ 2.0 Blue-Light Transilluminator (470 nm). For ITS-1 positive samples PCR was followed up by a RFLP assay for Leishmania strain identification, or amplicons sequenced using Macrogen Inc. service (Korea). Edition and alignment of the sequencer AB1 files were performed with Codon Code™ v 3.0.1 (CodonCode Corporation). Leishmania strain sequence homology was considered when the value retrieved by Blast (Basic Local Alignment Tool) was over 99% (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
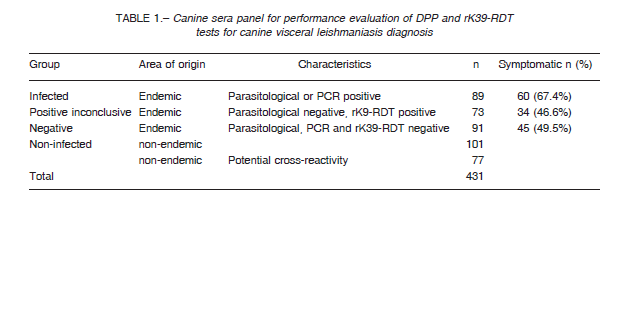
The panel (n = 431) involves the following sera samples categories: i) Infected dogs from the endemic area with positive parasitological test from popliteal lymph node smear or positive PCR (Origin: Posadas city, Argentina). ii) Positive inconclusive dogs from visceral leishmaniasis endemic area with negative parasitological test of popliteal lymph node smear but positive rK39-RDT (Posadas city). iii) Negative, dogs from the endemic area with negative parasitological test from popliteal lymph node smear, negative PCR, and negative rK39-RDT (Posadas city). iv) Non-infected, clinically healthy dogs from a non-endemic VL area (Buenos Aires city, Argentina). v)
Non-infected, other infections dogs from non-endemic areas, with infections of Trypanosoma cruzi (n = 50), Leptospira sp. (n = 13), Dirofilaria sp (n = 10), demodicosis by Demodex sp. (n = 3) and Brucella sp. (n = 1) (Table 1). Samples were further discriminated according the dog donor clinical status as: a) Asymptomatic, not presented any clinical sign of CVL disease. b) Symptomatic, presented lymphadenopathy together with other clinical sign, like onychogryphosis, cutaneous lesions, weight loss, conjunctivitis, alopecia or apathy. None dogs received leishmaniasis vaccine. The dogs were tested by optical parasitological diagnosis from popliteal lymph node smears and PCR from splenic aspirates as described in the section parasitological test, and DPP and rK39-RDT diagnostic test kits were used in accordance with manufacturer’s recommendations.
Test readers were blinded to the clinical status of the dog, and the results of other diagnostic tests.
Dogs were sampled during September 2013, in Oberá city (Argentina), an endemic area for CVL with antecedents of human visceral cases, in the neighborhoods of Villa Erasmie and Cien hectáreas, identified by the local agent of zoonosis as sectors with high density of canine presumptive cases.
Dwellings were selected by systematic random sampling, and in each dwelling all the dogs were sampled (n = 563). All sampled dogs were owned, and no samples from stray dogs were collected. The dogs were examined for clinical signs of CVL (categories as in the section above) and the symptoms recorded. None dogs received leishmaniasis vaccine. Popliteal lymph node aspirates and blood samples (venipuncture of the jugular or the cephalic) were collected regardless the clinical status, using disposable syringes and needles, sera were separated by centrifugation, and processed 3-4 h after collection for the rapid tests. DPP and rK39-RDT diagnostic test kits were used in accordance with manufacturer’s recommendations.
In addition, when the volume of the sample obtained from popliteal lymph node aspirates allowed it (n = 118), smears-parasitological direct tests and PCR were carried out.
For this last purpose the samples in 200 μl of phosphate buffer solution pH7.0 were kept cool in the field until they were frozen at -20 °C.
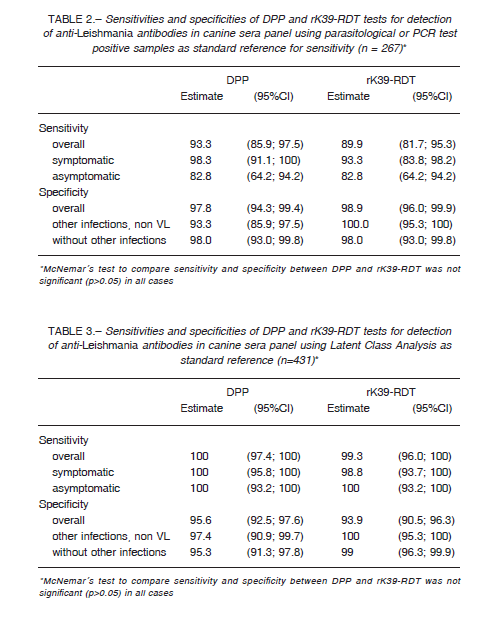
We compared DPP and rK39-RDT results of the sera panel between infected and non-infected groups (n = 267). In order to compute the sensitivity and specificity we used a positive parasitological and/or PCR results as true infected.
However, as a gold standard with high specificity but low sensitivity could underestimate the true specificity of the tests evaluated, we performed also a Latent Class Analysis (LCA), a statistical modeling technique recommended in absence of an acceptable gold standard, and previously used for canine
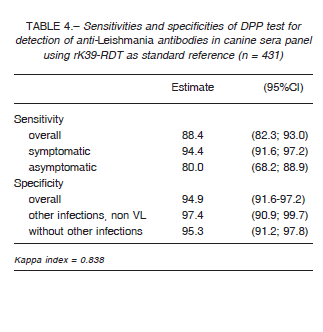
leishmaniasis 9-11. We used the results of the DPP, rK39-RDT and parasitological tests (n = 431) to define a non-observable (latent) variable indicating the true disease status of the sample 10. The goodness of fit of the statistical model was evaluated using the likelihood-ratio statistic G2 and entropy R2. DPP was also evaluated using rK39-RDT as reference standard (n = 431). The sensitivity and specificity for DPP and rK39-RDT of the sera panel and samples for field performance were estimated using 95% confidence interval (CI). In order to compare sensitivities and specificities the McNemar’s test was employed, with a significance level of 0.05. The agreement between the results obtained with the rapid tests was assessed using the Cohen’s Kappa statistic: no agreement (< 0), slight (0-0.20), fair (0.21-0.40), moderate (0.41-0.60), substantial (0.61-0.80), and perfect-almost perfect agreement (0.81-1).
The statistical analyses were performed using Stata™ software (version 13.1; Stata Corp, College Station, TX). Latent class analysis was performed using the LCA Stata Plugin™ (Version 1.2; University Park: The Methodology Center, Penn State).
Field procedures and handling of dogs were performed according to the protocol and informed approved by the Ethics Committee of the Fatala Chaben Institute and complies with National regulations and OIE recommendations. All dog owners agreed to include their dogs in the study and signed an informed consent form before sample collection.
Results
Table 2 summarizes the sera panel results of the two evaluated RDTs discriminated by the clinical status with the parasitological and/or PCR results as gold standard, while in Table 3 we used latent class analysis (LCA) as reference standard. Table 4 shows the results of DPP performance using rK39-RDT results as standard. Regarding to cross-reactivity, just one of sera diagnosed previously with Leptospira sp. infection reacted with DPP.
Table 5 shows the sensitivities and specificities of the sera sampled in Oberá city according to the clinical status and with the parasitological and/or PCR result as reference standard. The clinical status of the infected dogs (n = 143) were asymptomatic, (n = 77, 53.9%), symptomatic (n = 56, 39.2%), while 10 dogs (7.0%) were not clinically evaluated (n = 10, 7.0%). The PCR-RFLPs positive from splenic aspirates (n = 21) or sequenced positive PCR from splenic aspirates (n = 14) were identified as Leishmania infantum.
The kappa indexes between rK39-RDT and DPP was 0.838 in the sera panel (Table 4), while in field conditions it was 0.685 (Table 5).
The estimated overall prevalence of CVL in dogs from dwellings of two neighbors of high transmission in Oberá city was 28% (95% CI: 24.0-32.1). This value was computed assuming as ‘infected’ sero-reactive dogs
those with positive rK39-RDT and DPP, or dogs with one of them positive and positive by parasitological or PCR tests, and ‘non-infected’ dogs with both negative rK39 and DPP, or dogs with one of them negative and negative by parasitological and PCR tests (inconclusive results as rK39-DPP results/no parasitological nor PCR, negative parasitological without PCR, or negative PCR without parasitological were excluded (n = 52)).
Discussion
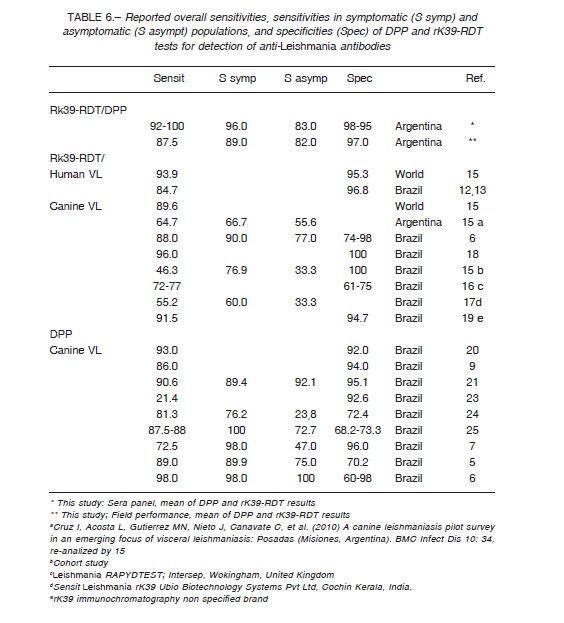
Using a panel of well characterized sera and parasitological or molecular diagnosis as reference standards, both rapid diagnostic tests evaluated showed an overall good sensitivity and specificity (Table 6). DPP and rK39-RDT had a very good agreement between them with a Kappa agreement index of 0.83, although the sensitivity of DPP taking into account rK39-RDT positives was 88.4% (95%CI: 82.3; 93.0).
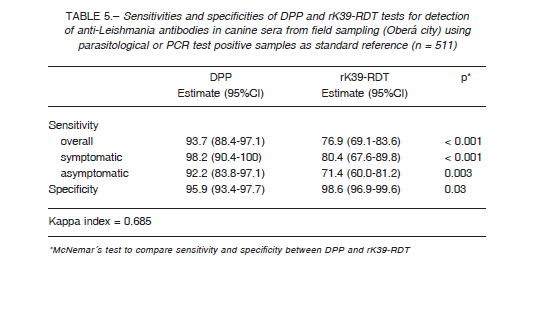
When the performance of the RDT tests was evaluated in the field, with a broader spectrum of cases, the specificity remains in the same range than the results with the sera panel (Table 6), but the overall sensitivity dropped due to the low performance of rK39-RDT ≈ 77%, and so the Kappa agreement index between the tests was 0.68, barely substantial. The results with dog populations in actual field scenarios are sensitive to the uncontrolled variables as the prevalence rate; in Brazil the Kappa index between DPP and rK39-RDT was 0.87 in a low transmission area (Spírito Santo State), but 0.54 in a high endemic area (Piauí State) 6. Further, the interpretation of tests by multiple operators, taking as negative some weak signals mainly from asymptomatic dog samples, could decrease the sensitivity of the tests. This fact highlights the operatordependent risk of bias when RDTs are used by agents in wide program activities, and so the need for standardized procedures, capacitation and quality control.
The performances obtained in this study are comparable with the results obtained by other authors (Table 6).
However, the sensitivity of RK39-RDT in much lower in some reports due to protocol differences as cohort studies or convenience sampling, and sera conservation procedures15, or the tests were performed with different brands of dipsticks 16, 17, 19. The disparate results reported for DPP were explained by cross-reactions mainly in asymptomatic dogs 7,21 and possible false positives in the gold standard 25.
As it was discussed above for the Kappa agreement index the specificity varies according the transmission scenarios from 74% to 98% for rK39-RDT and from 60% to 98% for DPP (Table 6) 6.
Therefore, the difference in the literature about CVL RDTs sensitivity, specificity and confidence interval amplitudes may be related both to methodological issues 13-16 and biological ones, besides the possible differences in parasite species/strains-geographical settings. According to the gold standard or the tests taken as true positive the sensitivity could vary from 15% to 26% 22. On the other hand, among the biological-based differences between studies, population and individual variables may modulate the performance of the test as genetic profile of dogs, socio-epidemiological context (life quality and immunocompromise), prevalence rate, time since infection, antibody titre, parasite load, clinical score and infectiousness 8, 15.
The cross-reactions reported are: rK39-RDT DiaMed-Vet-IT with Neospora caninum in 1 out of 9 sera, Hepatozoon canis 1/2 26, and human malaria 1/55 27; rK39-RDT InBios Ehrlichia sp. 1/3 and Trypanosoma cruzi 3/12 28; DPP with L. braziliensis 1/2 and 3/9, L. amazonensis 2/2 7, 29, Babesia sp. 4/9 21, Leptospira sp. 1/13 in our study.
Other authors did not found cross-reactions of DPP or rK39-RDT with Ehrlichia sp. or Babesia sp. 30. Therefore, besides the lack of information in same articles about differential diagnosis to discard double infections, there are also many inconsistencies about cross-reactions that may be due to the stage of infection-immunity of the control cases and common epitopes or precipitation of unspecific immunocomplexes. Anyway, there are more reports of cross-reactions with DPP than with rK39-RDT, and thus the reactivity with other species of Leishmania that also infect dogs in sympatric scenarios requires further investigation.
In conclusion, the performance of rK39-RDT and DPP for CVL diagnosis is comparable and acceptable at least for symptomatic dogs when tested in scenarios of visceral leishmaniasis recent southern spread in Argentina. CVL RDTs are more portable for point of care diagnosis, have lower costs and are simpler, have quicker results, improve the compliance of dog owners and the relationship with the communities 12. However, the statement of acceptability should be contextualized according the purpose of the test, from clinical diagnosis (individual-based), to seroprevalence studies (population-based) or outbreak control tools (decision making). In this sense, the evaluated RDTs do not discriminate between infection and immunity, involving pre-clinical and recently infected dogs, and chronic individuals who solved the infection but remain with immune memory 6. Therefore, both RDTs, are still acceptable for program dog population-based studies as spatial stratification, focus intervention and follow up, dog individual screening and confirmation of clinical presumptive CVL diagnosis in polysimptomatic dogs. However, the sensitivity to detect asymptomatic and even to discriminate among symptomatic the most infectious ones (core-transmitters/super-spreaders) is not enough for operational programs 15,31 , in order to target reservoir-based control interventions, mainly in high prevalence settings. Immunological tests in combination with other non-immunological based tests will be required for highly sensitive/specific diagnosis of infected dogs with L. infantum, for reservoir management both from public health and individual animal health perspectives.
Conflict of interest: None to declare
References
1. Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2010; 7: e35671.
2. PAHO-WHO 2018 Leishmaniasis Epidemiological Report of the Americas: Leishmaniasis Report
#6 – February, 2018. In: http://iris.paho.org/xmlui/bitstream/handle/123456789/34856/LeishReport6_eng.
pdf?sequence=1&isAllowed=y; accessed September 2019.
3. Salomón OD, Feliciangeli MD, Quintana MG, Afonso MM, Rangel EF. Lutzomyia longipalpis urbanisation and control. Mem Inst Oswaldo Cruz 2015; 110: 831-46.
4. Duthie MS, Lison A, Courtenay O. Advances toward diagnostic tools for managing zoonotic visceral leishmaniasis. Trends Parasitol 2018; 34: 881-90.
5. Figueiredo FB, Vasconcelos TCB, Madeira MF, et al. Validation of the Dual-path Platform chromatographic immunoassay (DPP® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Mem Inst Oswaldo Cruz 2018; 113: e180260.
6. Mendonça IL, Batista JF, Schallig H, et al. The performance of serological tests for Leishmania infantum infection screening in dogs depends on the prevalence of the disease. Rev Inst Med Trop Sao Paulo. 2017; 59: e39.
7. Grimaldi G Jr, Teva A, Ferreira AL, et al. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP(R) CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg 2012; 106: 54-9.
8. Schönian G, Nasereddin A, Dinse N, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis 2003; 47: 349-58.
9. Boelaert M, Aoun K, Liinev J, et al. The potential of latent class analysis in diagnostic test validation for canine Leishmania infantum infection. Epidemiology & Infection 1999; 123: 499-506.
10. Solca M da S, Bastos LA, Guedes CE, et al. Evaluating the accuracy of molecular diagnostic testing for canine visceral leishmaniasis using latent class analysis. PLoS One 2014; 9: e103635.
11. Fraga DB, Pacheco LV, Borja LS, et al. The rapid test based on Leishmania infantum chimeric rk28 protein improves the diagnosis of canine visceral leishmaniasis by reducing the detection of false-positive dogs. PLoS Negl Trop Dis 2016; 10: e0004333.
12. World Health Organization, 2011. Visceral leishmaniasis rapid diagnostic test performance. In: http://www.who.int/leishmaniasis/resources/9789241502238/en/ Accessed September 2019.
13. Cunningham J, Hasker E, Das P, et al. Visceral Leishmaniasis Laboratory Network. A global comparative evaluation of commercial immunochromatographic rapid diagnostic tests for visceral leishmaniasis. Clin Infect Dis 2012; 55: 1312-9.
14. Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ 2006; 333(7571):723.
15. Quinnell RJ, Carson C, Reithinger R, Garcez LM, Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis 2013;
7: e1992.
16. Reithinger R, Quinnell RJ, Alexander B, Davies CR. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J Clin Microbiol 2002; 40: 2352-6.
17. Silva DT, Starke-Buzetti WA, Alves-Martin MF, Paixão Mdos S, Tenório M da S, Lopes ML. Avaliação comparativa entre vários métodos para o diagnóstico da leishmaniose visceral canina. Rev Bras Parasitol Vet 2014; 23:179-86.
18. da Costa RT, França JC, Mayrink W, Nascimento E, Genaro O, Campos-Neto A. Standardization of a rapid immunochromatographic test with the recombinant antigens K39 and K26 for the diagnosis of canine visceral
leishmaniasis. Trans R Soc Trop Med Hyg 2003; 97: 678–82.
19. de Lima VM, Fattori KR, Michelin A de F, da Silveira Neto L, Vasconcelos R de O. Comparison between ELISA using total antigen and immunochromatography with antigen rK39 in the diagnosis of canine visceral leishmaniasis. Vet Parasitol 2010; 173: 330-3.
20. da Silva DA, Madeira M de F, Abrantes TR, Filho CJ, Figueiredo FB. Assessment of serological tests for the diagnosis of canine visceral leishmaniasis. Vet J 2013; 195: 252–3.
21. Laurenti MD, de Santana Leandro MV Jr, Tomokane TY, et al. Comparative evaluation of the DPP(®) CVL rapid test for canine serodiagnosis in area of visceral leishmaniasis. Vet Parasitol 2014; 205: 444-50.
22. Lopes EG, Sevá AP, Ferreira F, et al. Serological and molecular diagnostic tests for canine visceral leishmaniasis in Brazilian endemic area: one out of five seronegative dogs are infected. Epidemiol Infect 2017; 145: 2436-44.
23. Teixeira AIP, Silva DM, Vital T, et al. Improving the reference standard for the diagnosis of canine visceral leishmaniasis: a challenge for current and future tests. Mem Inst Oswaldo Cruz 2019; 114: e180452
24. de Santis B, Santos EGB, de Souza C da S, Chaves SA. Performance of DPP TM immunochromatographic rapid test (IRT) for canine visceral leishmaniasis: comparison with other serological methods in suspected dogs from Cuiabá, Mato Grosso State, Brazil. Braz J Vet Res Anim Sci 2013; 50: 198-5.
25. Schubach EY, Figueiredo FB, Romero GA. Accuracy and reproducibility of a rapid chromatographic immunoassay for the diagnosis of canine visceral leishmaniasis in Brazil. Trans R Soc Trop Med Hyg 2014;
108: 568-74.
26. Mettler M, Grimm F, Capelli G, Camp H. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent-antibody test, and two rapid tests (immuno chromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J Clin Microbiol 2005; 43: 5515-9.
27. Bangert M, Flores-Chávez MD, Llanes-Acevedo IP, et al. Validation of rK39 immunochromatographic test and direct agglutination test for the diagnosis of Mediterranean visceral leishmaniasis in Spain. PLoS Negl Trop Dis 2018; 12: e0006277.
28. Lemos EM, Laurenti MD, Moreira MA, et al. Canine visceral leishmaniasis: performance of a rapid diagnostic test (Kalazar Detect) in dogs with and without signs of the disease. Acta Trop 2008; 107: 205-7.
29. Paz GF, Rugani JMN, Marcelino AP, Gontijo CMF. Implications of the use of serological and molecular methods to detect infection by Leishmania spp. in urban pet dogs. Acta Trop 2018; 182: 198-201.
30. Krawczak F da S, Reis IA, Silveira JA, et al. Leishmania, Babesia and Ehrlichia in urban pet dogs: co-infection or cross-reaction in serological methods? Rev Soc Bras Med Trop 2015; 48: 64-8.
31. Mendonça IL, Batista JF, Werneck GL, Soares MRA, Costa DL, Costa CHN. Serological tests fail to discriminate dogs with visceral leishmaniasis that transmit Leishmania infantum to the vector Lutzomyia longipalpis. Rev Soc Bras Med Trop 2017; 50: 483-8.
