LEONARDO F. L. RIZZO 1, 2, DANIELA L. MANA 1, HÉCTOR A. SERRA 1, 3, KARINA DANILOWICZ 2
1 Dirección Médica, Química Montpellier SA, 2 División Endocrinología, Hospital de Clínicas José de San Martín, 3 Primera Cátedra de Farmacología, Facultad de Medicina, UBA, Buenos Aires, Argentina
Resumen La hiperprolactinemia puede asociarse con trastornos psiquiátricos en el contexto de dos escenarios: la hiperprolactinemia inducida por antipsicóticos y trastornos psiquiátricos surgidos por el tratamiento médico de la hiperprolactinemia. Ambas situaciones son particularmente comunes en la práctica clínica psiquiátrica y endocrinológica, aunque generalmente subestimadas o inadvertidas. El objetivo de este artículo es proporcionar herramientas de diagnóstico y tratamiento de la hiperprolactinemia asociada a trastornos psiquiátricos, para concientizar particularmente a psiquiatras y endocrinólogos a enfocar en conjunto el manejo apropiado de esta entidad.
Palabras clave: hiperprolactinemia, antipsicóticos, agonistas dopaminérgicos, trastornos de control de impulsos
Abstract Hyperprolactinemia may be associated with psychiatric disorders in the context of two scenarios: antipsychotic-induced hyperprolactinemia and psychiatric disorders arising from the medical treatment of hyperprolactinemia. Both situations are particularly common in psychiatric and endocrine clinical practice, albeit generally underestimated or unrecognized. The aim of this article is to provide tools for the diagnosis and treatment of hyperprolactinemia associated with psychiatric disorders to raise awareness, especially among psychiatrists and endocrinologists, so that these professionals can jointly focus on the appropriate management of this clinical entity.
Key words: hyperprolactinemia, antipsychotics, dopamine agonists, impulse control disorders
Dirección postal: Daniela Mana, Maza 578, 1220 Buenos Aires, Argentina
e-mail: daniela.mana@gmail.com
• Hyperprolactinemia may be associated with psychiatric disorders in the context of two scenarios: antipsychoticinduced hyperprolactinemia and psychiatric disorders arising from the medical treatment of hyperprolactinemia.
• It is important that psychiatrists and endocrinologists be aware and informed to approach together the management of hyperprolactinemia associated with psychiatric disorders in its different forms in order to avoid serious and devastating complications for the patient.
This review will discuss the relationship between hyperprolactinemia and psychiatric disorders from two different approaches: hyperprolactinemia secondary to the treatment of psychiatric disorders and psychiatric disorders induced by the medical treatment of hyperprolactinemia.
In both scenarios dopamine plays a leading role.
Dopamine is a neurotransmitter particularly important, involved in daily brain functioning (such as the control of motor function, motivation, and learning) and in several common disorders.
Three main dopaminergic pathways are described in the central nervous system 1:
– The nigrostriatal pathway, involved in control of motor function
– The mesocorticolimbic pathway, related to the socalled reward system, which regulates behaviour, pleasure and addiction
– The tuberoinfundibular dopaminergic pathway (TIDA), responsible for prolactin secretion
Alterations in these pathways will result in a variety of entities ranging from motor deficits (Parkinson’s disease), addictive and impulse control disorders and hyperprolactinemia1.
Prolactin physiology
Prolactin (PRL) is a 199-aa polypeptide hormone, with a molecular weight of 23 kDa, synthesized by lactotrophs in the adenohypophysis. PRL belongs to the large somatotrophin/PRL family of proteins that includes growth hormone (GH), placental lactogen, and PRL 2.
The recognition of the PRL receptor expression at numerous extra-pituitary sites including endometrial decidua, breasts, brain, ovaries, prostate, endothelial cells, lymphocytes, skin, adipose tissue and cochlea has opened up a wide range of potential functions. In any case, the most recognized role of PRL in humans is to induce lactation 2, 3.
The 23 kDa monomeric form is the main circulating variant of PRL (85%) and responsible for mediating its physiological actions. This variant acts through a membrane receptor belonging to the superfamily of type I cytokine receptors that are characterized by having a single transmembrane domain that transduces signals after phosphorylation of cytoplasmic kinases 2,3.
Other circulating forms of PRL are the “big” PRL and the “big big” PRL (macroprolactin). Big PRL is the dimer of monomeric form (50 kDa) and the “big big” PRL comprises high molecular (>150 kDa) complexes formed by 23 kDa PRL and IgG autoantibodies that can be found in different degrees in immunoassays for PRL. It seems that these forms of PRL have minimal or any biological activity 4,5.
Secretion of PRL by the adenohypophysis has a circadian rhythm with higher levels during sleep and lower during wakefulness4. Increased levels are also seen during ovulation. Synthesis and secretion of PRL are both regulated by releasing and inhibiting factors. PRL inhibiting factors include dopamine, gamma-aminobutyric acid (GABA), and somatostatin. Dopamine is the main regulator of PRL secretion through its D2 receptor, and unlike other pituitary hormones, the inhibiting component is dominant.
Consequently, PRL level will increase if the blood flow of the pituitary stalk is altered or if dopamine synthesis is inhibited or in case of receptor blockade 2, 5.
Among the PRL releasing factors can be mentioned TRH (thyrotropin-releasing hormone), VIP (vasoactive intestinal peptide), serotonin, opioid peptides, GHRH (growth hormone-releasing hormone), neurotensin, oxytocin, vasopressin, galanin, and estrogens 2, 4.
Dopamine is released through the axons of the TIDA pathway in the median eminence. These axons are projected from the dorsomedial portion of the arcuate nucleus and the lower portion of the ventromedial nucleus of the hypothalamus.
There are five types of dopamine receptors that belong to the family of G-protein-coupled receptors. D1 and D5 receptors couple to Gs protein and stimulate the synthesis of cyclic AMP. Meanwhile, D2, D3 and D4 receptors couple to Gi protein and inhibit the synthesis of cyclic AMP and inositol phosphate metabolism, decreasing the intracellular calcium mobilization and transportation through its channels 6.
The dopamine action on the adenohypophysis is exerted through the D2 and D4 receptors located in the lactotroph membrane, inhibiting – tonically and directly -PRL gene transcription, PRL synthesis and secretion and lactotroph proliferation 2, 4-6.
Hyperprolactinemia
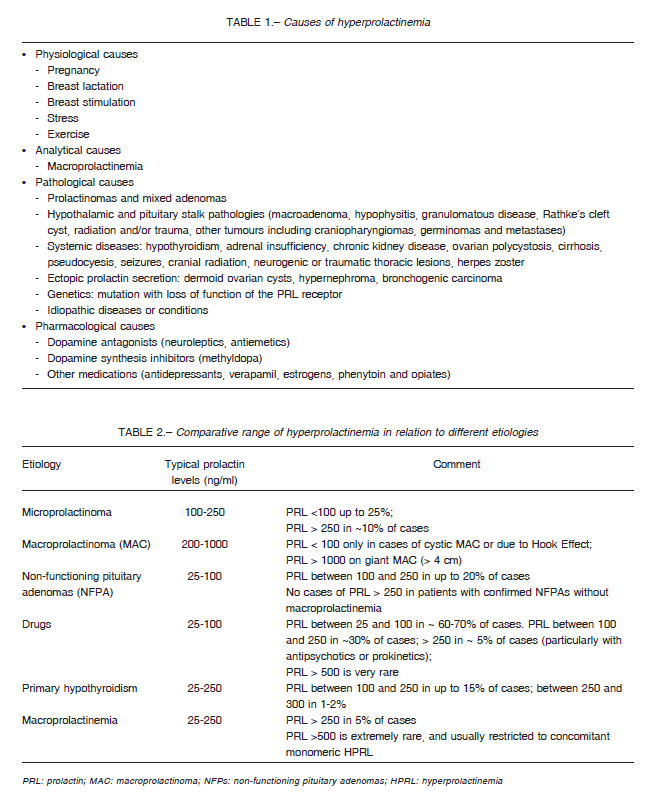
It is the most common endocrine disorder of the hypothalamic- pituitary unit. The causes of hyperprolactinemia can be physiological, analytical, pathological and pharmacological (Table 1)3, 7, 8.
Serum PRL levels are higher in women than in men and hyperprolactinemia is diagnosed when the PRL levels are higher than 25 ng/ml in females and higher than 20 ng/ml in males 7.
Hyperprolactinemia occurs at any age and its prevalence varies around 1% regarding adult population (0.2% in men and 1% in women), and 5% in cases of women with infertility 9-11. Hypogonadotrophic hypogonadism and galactorrhea are the most characteristic signs and symptoms of PRL excess and will be discussed below.
Hyperprolactinemia secondary to the treatment of psychiatric disorders
Drug-induced hyperprolactinemia is the most frequent cause of non-physiological hyperprolactinemia 8. In fact, an epidemiological study in Scotland evaluated 1301 cases of hyperprolactinemia (excluding pregnancy) and the results showed that 45.9% of the cases were druginduced 12.
A considerable number of drugs cause PRL elevations through different mechanisms 13, 14 that are listed below:
– Increased transcriptional activity of PRL gene: estrogens
– Dopamine receptor antagonism: risperidone, haloperidol, phenothiazines, metoclopramide, domperidone, sulpiride
– Dopamine depletion: reserpine, methyldopa
– Hypothalamic dopamine synthesis inhibition: verapamil, heroin, morphine
– Serotonin reuptake inhibition: opiates, fluoxetine, tricyclic antidepressants, monoamine-oxidase inhibitors
Usually the range of drug-induced hyperprolactinemia varies from 25 to 100 ng/mL. However, levels > 100 ng/mL can be observed, especially with the use of neuroleptics (risperidone, haloperidol, chlorpromazine) and antiemetics (metoclopramide and domperidone) 15, 16. Table 2 shows the comparative range of hyperprolactinemia due to different etiologies.
As regards in particular to antipsychotics, butyrophenones and phenothiazines may induce PRL excess in 50-90% of treated patients, while risperidone in 70-100% of cases 7, 17. In relation with risperidone, PRL serum levels even greater than 300 ng/ml have been reported 8,18.
Different classes of antidepressants, including monoamine oxidase inhibitors, tricyclic antidepressants, and selective serotonin reuptake inhibitors induce hyperprolactinemia mainly via serotoninergic pathways. Hyperprolactinemia is typically mild and rarely symptomatic 2, 4.
Mechanisms of antipsychotic-induced hyperprolactinemia
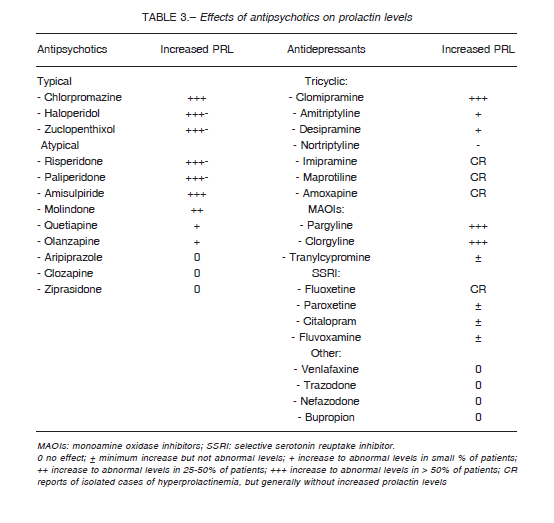
Antipsychotics can be classified into those with great potential for PRL elevation, and those that modify PRL to a lesser degree or even decrease its level. Explanations for these differences include variations in the D2 receptor dissociation rate, ability to cross the blood-brain barrier, presence of D2 receptor polymorphisms (Taq1A genotype) 19, and degree of serotoninergic inhibition4. The effects of antipsychotics on prolactin levels are variable as can be seen in Table 3.
In addition, the tenor of antipsychotic-induced hyperprolactinemia also depends on the affinity rate and the duration of the D2 receptor blockade 20, 21. Haloperidol, for example, blocks D2 receptors for a period of more than 24 hours compared to quetiapine, which shows a milder and more transient effect. Besides, quetiapine and clozapine are rapidly dissociated from D2 receptors and both act as antagonists of 5-HT2 serotonergic receptors, causing modest increases in PRL levels 21, 22.
In contrast to typical antipsychotics, the atypical ones induce milder PRL elevations due to less affinity for the D2 receptor and greater blockade on the 5-HT2A receptor.
The exception to the rule includes risperidone, paliperidone and amisulpiride that usually cause significant PRL elevation 17, 18. In fact, risperidone, as expressed before, has the greatest impact due to its high antagonistic activity on D2 and 5HT2 receptors 17,18,20, 22. Both oral and depot forms of risperidone and paliperidone increase PRL in a dose-dependent manner.
The threshold for the D2 receptor blockade at which the increase of PRL level occurs would range between 65-80% 21. PRL levels increase within a few days after initiation of antipsychotic medication, usually within the first 2 to 3 weeks with a wide range of 7 to 75 days 6.
Aripiprazole deserves a special mention for its particular mechanism of action. It behaves as a partial D2 and 5-HT1A receptors agonist Consequently, the drug does not cause a significant increase of PRL concentration and even reduces it when associated with another antipsychotic 23, 24.
Finally, estrogens enhance PRL secretion via different mechanisms including upregulation of PRL gene expression, raise sensitivity to TRH, downregulation of pituitary dopamine receptors expression, and stimulation of lactotroph hyperplasia 4. For this reason, antipsychotic-induced hyperprolactinemia is more common in adolescents and premenopausal women17, 18.
Pathological consequences of hyperprolactinemia
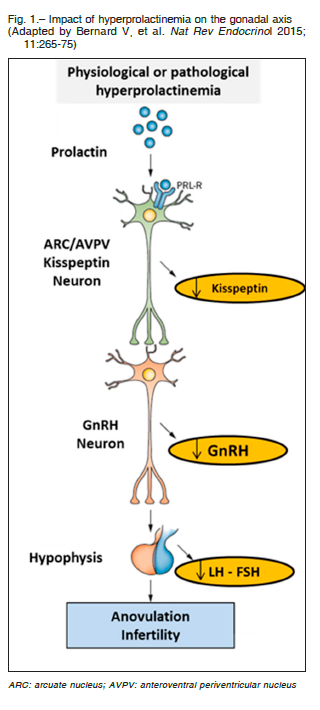
The clinical impact does not differ from other causes of hyperprolactinemia but hypogonadotrophic hypogonadism deserves special consideration. Recent evidences indicate that hyperprolactinemia causes lower production of kisspeptin at the hypothalamic level reducing the hypothalamic secretion of GnRH (gonadotrophin-releasing hormone) and consequently the synthesis and secretion of pituitary gonadotrophins (LH and FSH) with a loss of gonadal stimulation and infertility 3 (Fig. 1).
In model mice with hyperprolactinemia as well as in women with hypogonadotrophic amenorrhea caused by hyperprolactinemia, GnRH and gonadotropin secretion and ovarian cyclicity were restored by kisspeptin administration25, 26.
Thus, hypogonadism induced by PRL excess is clinically characterized in women by menstrual disorders such as oligoamenorrhea and galactorrhea; hirsutism, acne and vaginal dryness may also be present3, 7. Menstrual disorders usually occur with PRL levels greater than 50 ng/ml 27. PRL levels between 30 to 50 ng/ml can cause a deficient luteal phase also being a cause of infertility.
Galactorrhea (either spontaneous or deliberate) is a common manifestation in premenopause (up to 90%), but occurs less frequently in menopause owing to the lack of estrogenic effect on the breasts28.
In men PRL excess can be accompanied by gynecomastia (very rarely by galactorrhea), sexual dysfunction and infertility. In both sexes, hyperprolactinemia may induce decreased libido, osteopenia or osteoporosis, and fracture risk3, 7, 28.
It is important to keep in mind those patients whose first psychotic outbreak occurs in children or adolescents (< 18 years of age) and who will require antipsychotic treatment for several years29, 30. This population is at increased risk of experiencing adverse effects in the short (< 6 months) and medium-term (6-12 months), such as sedation, extrapyramidal symptoms, hypogonadism, galactorrhea, delayed puberty, weight gain and dyslipidemia18, 30.
These metabolic alterations should be explained because hyperprolactinemia has been associated with an increase in food intake, weight gain and obesity. Moreover, PRL excess has been shown to reduce glucose tolerance and hyperinsulinemia in humans31.
The effects of hyperprolactinemia will be exacerbated on the long-term (> 12 months) with deleterious effects especially on bone tissue. Its consequences will be the lack of reaching peak bone mass and the resulting risk of osteoporosis and fractures later in life18, 32.
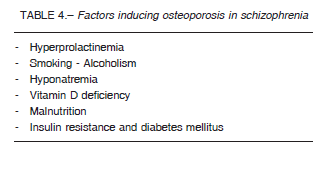
While osteoporosis is a known complication of hypogonadism in general and also as mentioned before, of hypogonadism secondary to hyperprolactinemia, many other factors are involved in the increased risk of osteoporosis in the psychotic patient32. Table 4 shows the different factors involved in the development of osteoporosis in schizophrenia. These factors include hyponatremia, which can increase the risk of fractures through two mechanisms. The former arises from an increase in falls triggered by gait disorders resulting from a decrease in brain glutamate concentration, which is the neurotransmitter responsible for gait. Even asymptomatic chronic hyponatremia increases falls (21% vs. 5% in normal controls).
This happens because chronic hyponatremia stimulates osteoclastogenesis and bone resorption34-36. Since one third of total body sodium is stored in bone, in response to hyponatremia, sodium is mobilized outside the bone tissue similar to what is observed in calcium deprivation.
Causes of hyponatremia in schizophrenia can be attributed to psychogenic polydipsia (dilutional hyponatremia) that affects 6- 20% of patients; increased antidiuretic hormone (ADH) secretion induced by psychosis per se or produced by drugs such as haloperidol, phenothiazines, MAOIs, selective serotonin reuptake inhibitors and amitriptyline33, 34.
Hyperprolactinemia would also be a risk factor for obesity-related metabolic syndrome37, insulin resistance and diabetes mellitus through apparently by way of a direct effect on pancreatic beta cells3, 38.
Approach to the patient with antipsychoticinduced hyperprolactinemia
Determination of serum PRL
Serum PRL levels should be measured in patients receiving any drug that may cause serum PRL increases, and in particular in those patients with signs and symptoms of hyperprolactinemia. Once these serum PRL elevations
are confirmed, it is advisable to temporarily discontinue the drug intake for at least 72 hours and repeat the measurement7.
Specifically, with oral antipsychotics, PRL levels tend to normalize within 48 to 96 hours after the last dose8,15. However, elevated levels can persist as long as about 3 weeks depending on drug half-life and storage in fatty tissue39.
This practice, however, is not commonly applied to psychotropic drugs as there is insufficient data on the consequences of discontinuing its use for a few days to measure PRL and the risk for exacerbation of the psychiatric condition7.
Therefore, it would be advisable to have baseline PRL levels, as well as glucose and lipid profiles before prescribing an antipsychotic, given the potential of these drugs to induce insulin resistance and metabolic syndrome.
During treatment, it is suggested to monitor prolactin levels two to three weeks after psychotropic drug initiation, and then regularly every two to three months. It is also advisable to check PRL levels in case it is necessary to increase the dose or to replace the drug with an alternative psychotropic medication6, 40.
It is also important to consider the presence of macroprolactinemia (analytical hyperprolactinemia) in particular in asymptomatic patients with hyperprolactinemia who are receiving antipsychotics, as this condition may also occur in these cases and may induce clinical and diagnostic confusion5,8. The incidence of macroprolactin varies among different series, but it is estimated to occur in about 10-25% of cases of hyperprolactinemia5.
Macroprolactinemia is detected by the polyethylene glycol (PEG) precipitation test in particular and, if available, by gel chromatography. With the PEG test, the higher molecular weight forms of PRL are removed by precipitation leading to the residual monomeric form in the sample supernatant. If the recovery of monomeric PRL after precipitation with PEG is less than 40% of the initial total value, then macroprolactin is the predominant variant present of immunoreactive PRL5, 39.
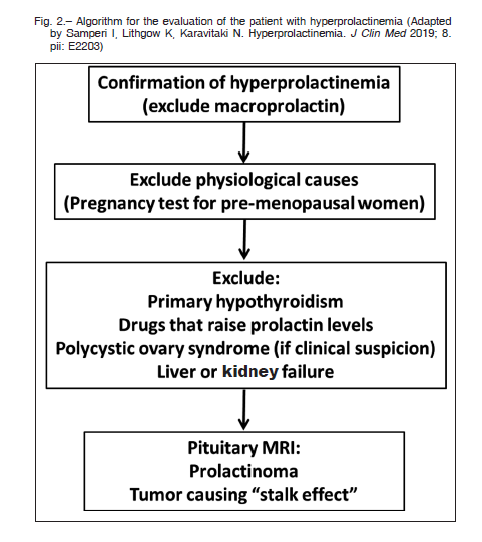
Other causes of hyperprolactinemia to be excluded are: pregnancy (in all premenopausal women), primary hypothyroidism, polycystic ovary syndrome (especially in presence of hyperandrogenism) and liver and kidney failure4.
Imaging studies
Pituitary magnetic resonance imaging (MRI) with gadolinium is the best method for visualizing the presence of abnormalities in the sellar region7. There is no PRL level that can differentiate a lactotroph tumor from drug-induced hyperprolactinemia, although a value > 200 ng/ml is suggestive of macroprolactinoma.
It is relevant for decision-making to know the previous PRL level and the gynecological (regular cycles) or andrological (sexual function) records of the patient prior to the treatment with psychotropic medication.
If the drug cannot be discontinued or replaced by another psychotropic (concerning exclusively to the psychiatrist) and the presence of hyperprolactinemia was not coincident with the treatment initiation, it is advisable to perform MRI studies of the sellar region7,15.
Besides, a prolactinoma or any sellar lesion may coexist in patients treated with antipsychotics, fact that may exacerbate the serum PRL level.
It is important to be aware of the presence of nonfunctioning adenomas or other clinically silent pituitary lesions reported in about 10% of MRI images that may not correspond to a prolactinoma, even in the presence of altered serum PRL levels39. Figure 2 proposes an algorithm for the evaluation of patients with hyperprolactinemia
Therapeutic approach to the patient with antipsychotic-induced hyperprolactinemia
Management of this situation requires a joint therapeutic approach with the psychiatrist and the endocrinologist.
Hyperprolactinemia caused by antipsychotics is an underestimated, unrecognized or ignored adverse effect in spite of the potentially serious complications it causes5,17, 19.
The approach to this situation is controversial. The first option would be drug discontinuation, difficult to apply in patients under treatment with antipsychotics.
Patients with asymptomatic hyperprolactinemia simply require periodical clinical and laboratory monitoring to evaluate the appearance of symptoms related to hyperprolactinemia7.
This approach should be reconsidered in the case of women of childbearing age with a desire for pregnancy, since excess PRL, even when mild, may be a cause of infertility.
In symptomatic patients with drug-induced hyperprolactinemia, one could try to reduce the dose of the antipsychotic, although this can result in an aggravation of the psychiatric condition. It may also be ineffective for the control of hyperprolactinemia, given that it is not always possible to normalize PRL levels with the reduction of the psychotropic dose. Another proposed option is to switch to another antipsychotic with less impact on PRL levels, such as quetiapine, but difficult to accomplish if the patient is well controlled with the initial psychiatric treatment6,17, 18.
Adjuvant therapy with aripiprazole, a partial dopamine agonist, has proven efficacy in reducing hyperprolactinemia.
However, two possibilities are worth considering; one that the primary anti-psychotic efficacy may be decreased and second a higher risk of developing side effects when adding another psychotropic23,41, 42.
For those patients whose psychiatric state is well controlled but present signs of hyperprolactinemia such as hypogonadism or low bone mineral density, the Endocrine Society recommends replacement therapy with estrogens for women and testosterone for males7. If the impact on bone mass is significant or if hormone replacement therapy is contraindicated, vitamin D and calcium treatment should be implemented together with bisphosphonates, if necessary7, 43.
Adding a dopamine agonist such as cabergoline is not recommended given the specific risk of exacerbation of psychosis8,44, 45.
Cabergoline at lower doses could only be considered in very well selected cases such as fertility desire or contraindicated hormonal replacement therapy, and if the aforementioned alternatives could not be undertaken or were ineffective. This therapeutic approach will require close monitoring by the psychiatrist 7,8.
Psychiatric disorders induced by the medical treatment of hyperprolactinemia
Once pregnancy, hypothyroidism and drugs have been ruled out, the most frequent cause of chronic hyperprolactinemia is a prolactin-secreting tumor7,8. Prolactinomas are the most common pituitary adenomas, accounting for approximately 50% of all pituitary tumors. They are classified according to their size in microprolactinomas < 1 cm and macroprolactinomas ≥ 1 cm, and in giant prolactinomas when measuring > 4 cm. These tumors most commonly affect women, especially between the ages of 25 and 44 years old, with a female to male ratio of 20:1 for microprolactinomas. The ratio between men and women tends to be similar for macroprolactinomas, although these tumors are usually larger in males46.
Dopamine agonists (DA) have become the first-line therapy for prolactinomas. They induce not only the reduction of PRL secretion but also of the tumor size.
Commercially available DAs are bromocriptine, cabergoline and quinagolide. In Argentina, cabergoline is the only DA commercially available. Bromocriptine and cabergoline are ergot derivatives with agonist activity on the D2 receptor. Cabergoline is a more selective agonist with a longer half-life that allows one to two weekly doses, usually ranging from 0.5 to 3 mg/week. Quinagolide is a non-ergot derivative that also causes agonist action at D2 receptors. Current guidelines recommend cabergoline as the first-line DA given its superior efficacy to bromocriptine, its improved tolerance and convenient dosage7,13.
DA therapy is often prolonged and if PRL levels remain normal for at least two years and the tumor is no longer visualized on MRI studies, the drug can be discontinued.
Another strategy if the tumor reduced its size by more than 50% and is more than 5 mm away from the optic chiasm, is to gradually taper the DA dose to the minimum concentration required to maintain both a normal PRL level and control the tumor volume or even to discontinue the therapy46-48.
The most frequent adverse effects are nausea and/or vomiting, postural hypotension that may be accompanied by dizziness and syncope, headaches, Raynaud’s phenomenon and more rarely nasal congestion, flushing and cramps. These adverse effects are related to activation of D1 and 5HT1 receptors. High dosages of DA, as used for Parkinson’s disease, have been associated with valvulopathy caused by activation of the 5HT2B receptors.
Until present, it seems that risk of valvulopathy would not be significant with dosages usually used in patients with prolactinomas49, 50, with a very low incidence of 0.11% according to some series51. However, if the cabergoline dosage used is higher than 2 mg/week, an annual echocardiogram is recommended50, 52.
More rarely, DA can induce mood swings and psychosis in susceptible patients53.
Other classically reported psychiatric symptoms in patients with Parkinson’s disease treated with high DA doses are impulse control disorders that have been reported most recently in patients with prolactinomas, treated with even low DA doses54.
Impulse control disorders
Impulse control disorders (ICD) are defined as failure to resist an impulse or temptation to perform a harmful act on oneself or others. These disorders include addictions to gambling, sex, food, compulsive shopping, kleptomania, intermittent explosive disorder, vigorexia, and punding.
The term punding was introduced by Swedish psychiatrist Carl Rylander in 1968 in connection with consumers of phenmetrazine (sympathomimetic amphetamine). Punding is defined as a complex, prolonged, unproductive, and stereotypical behavior that is often associated with ICDs. It is characterized by compulsion and fascination for performing non-purpose repetitive mechanical tasks such as assembling and disassembling devices – watches, computers, collecting or classifying several objects simultaneously, emptying and rearranging household drawers and shelves, among others55-57.
The physiopathological mechanism of these disorders is caused by an interaction between dopamine agonist and D3 receptors at the level of the mesocorticolimbic system, responsible for the processes that regulate behavior, pleasure and addiction 56.
The prevalence of ICDs is set at about 8% of the general population57. The percentage increases considerably when ICDs caused by DAs are considered as well. In a retrospective data analysis published by the Food and Drug Administration (FDA) on the Adverse Effects Reporting System between 2003 and 2012, the prevalence of ICD induced by DAs in patients with Parkinson’s disease was 61.7%, in those with restless leg syndrome 24%, and in those with hyperprolactinemia 3.5%58. ICDs were more common in patients treated with selective D3 agonists such as pramipexole and ropinirole, which would explain a higher frequency in Parkinson’s disease.
Thus, the occurrence of ICD in patients with prolactinomas treated with DAs such as bromocriptine or cabergoline has been considered an unusual event until recently, with a prevalence of about 3-4% and has been preferably associated with the use of high doses of DA.
The first case of gambling addiction was reported in 2007 by a 47-year-old patient treated with cabergoline, 0.25 mg/week59.
However, more recent case series have described a prevalence of up to 17% in patients with prolactinomas treated mostly with cabergoline60. Among different case series, the most frequent ICD was hypersexuality with a greater incidence in males55-66. It is worth noting that about 33% of affected patients may develop multiple types of ICDs while treated with DAs57, 63, 64.
Other infrequent psychiatric disorders associated with the use of DAs are major depressive disorder, hallucinations, sleep disorders, manic episodes and psychosis; which generally resolve with the discontinuation of treatment57.
Some risk factors have been established for developing ICD such as the male sex, an early onset of the disease (particularly observed in relation to Parkinson’s disease), personality traits (depression, aggressiveness, impulsiveness), history of psychiatric disorders, alcohol consumption or other addictions1, 56, 57.
Consequently, before prescribing DAs, it is advisable to enquire about the patient’s habits and personality traits that may relate to an increased possibility of developing these complications55-57.
Once the treatment has been initiated, during follow-up consultations, it will be of importance to inquire the patient, and if possible their relatives or caregivers, about the onset of symptoms compatible with ICDs since the patients themselves may either not be aware of the symptoms or hide the situation57.
ICDs may occur at any time after the DA treatment initiation (early or late) requiring periodic monitoring. Some questionnaires and psychometric tests can be used: Barratt Impulsiveness Scale and the Behavioural Inhibition/ Activation System (BIS/BAS), Depression Anxiety Stress Scale (DASS21), Questionnaire of Impulsive Control Disorders in Parkinson’s disease (QUIP-S), Hypersexual Behaviour Inventory (HBI), among others55-57, 66.
Upon occurrence, ICDs should be managed together with the psychiatrist and the initial treatment will be to either reduce the DA dose or discontinue the drug. It is no use substituting one DA for another.
Burback describes the case of a 32-year-old patient with microprolactinoma who developed an acute manic episode with weekly doses of 0.5 mg of cabergoline. The manic episode quickly reversed after cabergoline was discontinued and replaced with aripiprazole 5 mg/day which was sufficient to keep the PRL level suppressed and to stabilize the psychiatric state67. Tumor size remained stable over 15 months of follow-up. Therefore, aripiprazole would be a very good alternative for the control of hyperprolactinemia for those patients with microprolactinomas who develop DA-induced psychiatric disorders57. Another option to be considered in these situations is transphenoidal pituitary surgery13, 57.
The complications of DA-induced ICDs can be serious and catastrophic for the life of the patient, with great impact on their family/social and professional life, financial decisions, and mental and physical health.
In a recent study, only 37% of the interviewed patients with hyperprolactinemia (n= 51) had been warned about the relationship between ICDs and DA therapy66.
Although to date there are no reported cases of ICDs in patients treated with DAs for other endocrinopathies such as acromegaly, Cushing’s disease and non-functioning pituitary tumours it is advisable to consider their possible development when prescribing CBG.
In conclusion, in patients treated with antipsychotics, prescribing dose, treatment duration, antipsychotic potency (D2 receptor blockade), age and sex are contributing factors to the level of hyperprolactinemia, occurring more frequently in adolescent and premenopausal women.
It would be advisable to measure the PRL level before treatment initiation and then periodically, in every patient treated with antipsychotics. Antipsychotic-induced hyperprolactinemia is an underestimated, unrecognized or ignored side effect that requires treatment in many cases.
The consequences of hyperprolactinemia can be serious and compromise adherence to treatment. Management of hyperprolactinemia requires a joint therapeutic approach between the psychiatrist and the endocrinologist.
Although, ICDs have been considered relatively uncommon adverse effects in the endocrinology-related field, in recent years, with the use of DAs, the number of reported cases has increased considerably. It is prudent and advisable that prior to treatment initiation, physicians warn and educate patients and/or their families or caregivers about the potential adverse effects of DAs.
ICDs can occur unpredictably, at any age, affect both sexes, also with low DA doses, and usually disappear when ceasing treatment.
Finally, the aim of this review is to inform psychiatrists and endocrinologists of the need for joint management of hyperprolactinemia associated with psychiatric disorders in its different forms in order to avoid serious and devastating complications for the patient.
Conflict of interest: Leonardo F. L. Rizzo is the Medical Director of Química Montpellier S.A. Daniela Mana and Héctor Serra are Medical Consultants for Química Montpellier S.A.
References
1. Grall-Bronnec M, Victorri-Vigneau C, Donnio Y, et al. Dopamine Agonists and Impulse Control Disorders: A Complex Association. Drug Saf 2018; 41:19-75.
2. Binart N. Prolactin. In: Melmed S (ed) The Pituitary, 4th ed. San Diego; Academic Press, 2017, p 129-61.
3. Bernard V, Young J, Chanson P, Binart N. New insights in prolactin: pathological implications. Nat Rev Endocrinol 2015; 11: 2656-75.
4. Samperi I, Lithgow K, Karavitaki N. Hyperprolactinemia. J Clin Med 2019; 8: pii: E2203.
5. Saleem M, Martin H, Coates P. Prolactin biology and laboratory measurement: an update on physiology and current analytical issues. Clin Biochem Rev 2018; 39: 3-16.
6. Milano W, Colletti C, Capasso A. Hyperprolactinemia induced by antipsychotics: from diagnosis to treatment approach. Endocr Metab Immune Disord Drug Targets 2017; 17: 38-55.
7. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011; 96: 273-88.
8. Vilar L, Freitas Vilar C, Lyra R, Freitas MDC. Pitfalls in the diagnostic evaluation of hyperprolactinemia. Neuroendocrinology 2019; 109: 7-19.
9. Chanson P, Maiter D. Prolactinoma. In: Melmed S (ed) The Pituitary, 4th ed. San Diego: Academic Press, 2017, p 467-514.
10. Kars M, Souverein PC, Herings RM, et al. Estimated ageand sex-specific incidence and prevalence of dopamine agonist-treated hyperprolactinemia. J Clin Endocrinol Metab 2009; 94: 2729-34.
11. Souter I, Baltagi LM, Toth TL, Petrozza JC. Prevalence of hyperprolactinemia and abnormal magnetic resonance imaging findings in a population with infertility. Fertil Steril 2010; 94:1159-62.
12. Soto-Pedre E, Newey PJ, Bevan JS, Greig N, Leese GP. The epidemiology of hyperprolactinaemia over 20 years in the Tayside region of Scotland: The Prolactin Epidemiology, Audit and Research Study (PROLEARS). Clin Endocrinol (Oxf) 2017; 86: 60-7.
13. Vilar L, Abucham J, Albuquerque JL, et al. Controversial issues in the management of hyperprolactinemia and prolactinomas – An overview by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch Endocrinol Metab 2018; 62: 236-63.
14. La Torre D, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag 2007; 3: 929-51.
15. Molitch ME. Drugs and prolactin. Pituitary 2008; 11: 209-18.
16. Fleseriu M. Drugs and pituitary function. In: Melmed S (ed). The Pituitary 4th ed. San Diego: Academic Press, 2017, p 383-96.
17. Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 2014; 28: 421-53.
18. Montejo ÁL, Arango C, Bernardo M, et al. Multidisciplinary consensus on the therapeutic recommendations for iatrogenic hyperprolactinemia secondary to antipsychotics. Front Neuroendocrinol 2017; 45: 25-34.
19. Miura I, Zhang JP, Hagi K, et al. Variants in the DRD2 locus and antipsychotic-related prolactin levels: A metaanalysis. Psychoneuroendocrinology 2016; 72: 1-10.
20. Ajmal A, Joffe H, Nachtigall LB. Psychotropic-induced hyperprolactinemia: a clinical review. Psychosomatics 2014; 55: 29-36.
21. Tsuboi T, Bies RR, Suzuki T, et al. Hyperprolactinemia and estimated dopamine D2 receptor occupancy in patients with schizophrenia: analysis of the CATIE data. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45: 178-82.
22. Vyas NS, Patel NH, Nijran KS, Al-Nahhas A, Puri BK. The use of PET imaging in studying cognition, genetics and pharmacotherapeutic interventions in schizophrenia. Expert Rev Neurother 2011; 11: 37-51.
23. Hoffer ZS, Roth RL, Mathews M. Evidence for the partial dopamine-receptor agonist aripiprazole as a first-line treatment of psychosis in patients with iatrogenic or tumorogenic hyperprolactinemia. Psychosomatics 2009; 50: 317-24.
24. De Berardis D, Fornaro M, Serroni N, et al. Treatment of antipsychotic-induced hyperprolactinemia: an update on the role of the dopaminergic receptors D2 partial agonist aripiprazole. Recent Pat Endocr Metab Immune Drug Discov 2014; 8: 30-7.
25. Sonigo C, Bouilly J, Carré N, et al. Hyperprolactinemiainduced ovarian acyclicity is reversed by kisspeptin administration. J Clin Invest 2012; 122: 3791-5.
26. Millar RP, Sonigo C, Anderson RA, et al. Hypothalamicpituitary- ovarian axis reactivation by kisspeptin-10 in hyperprolactinemic women with chronic amenorrhea. J Endocr Soc 2017; 1: 1362-71.
27. Takechi K, Yoshioka Y, Kawazoe H, et al. Psychiatric patients with antipsychotic drug-induced hyperprolactinemia and menstruation disorders. Biol Pharm Bull 2017; 40: 1775-8.
28. Capozzi A, Scambia G, Pontecorvi A, Lello S. Hyperprolactinemia: pathophysiology and therapeutic approach. Gynecol Endocrinol 2015; 31: 506-10.
29. Ben Amor L. Antipsychotics in pediatric and adolescent patients: a review of comparative safety data. J Affect Disord 2012; 138 Suppl: S22-30.
30. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents.
J Am Acad Child Adolesc Psychiatry 2006; 45: 771-91.
31. Auriemma S, Granieri L, Galdiero M, et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology 2013; 98: 299-310.
32. Takahashi T, Uchida H, John M, et al. The impact of prolactin-raising antipsychotics on bone mineral density in patients with schizophrenia: findings from a longitudinal observational cohort. Schizophr Res 2013; 147: 383-6.
33. De Hert M, Detraux J, Stubbs B. Relationship between antipsychotic medication, serum prolactin levels and osteoporosis/ osteoporotic fractures in patients with schizophrenia: a critical literature review. Expert Opin Drug Saf 2016; 15: 809-23.
34. Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem 2011; 286: 10864-75.
35. Verbalis JG, Barsony J, Sugimura Y, et al. Hyponatremiainduced osteoporosis. J Bone Miner Res 2010; 25: 554-63.
36. Usala RL, Fernandez SJ, Mete M, et al. Hyponatremia is associated with increased osteoporosis and bone fractures in a large US Health System Population. J Clin Endocrinol Metab 2015; 100: 3021-31.
37. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of antipsychotic induced weight gain on health and mortality rate. Psychiatry Res 2001; 101: 277- 88.
38. Carré N, Binart N. Prolactin and adipose tissue. Biochimie 2014; 97: 16-21.
39. Petersenn S. Biochemical diagnosis in prolactinomas: some caveats. Pituitary 2020; 23: 9-15.
40. Grigg J, Worseley R, Thew C, Gurvich C, Thomas N, Kulkarni J. Antipsychotic-induced hyperprolactinemia: Synthesis of world-wide guidelines and integrated recommendations for assessment, management and future research. Psychopharmacology 2017; 234: 3279-97.
41. Zheng W, Cai DB, Yang XH, et al. Adjunctive aripiprazole for anti-psychotic related hyperprolactinemia in patients with first-episode schizophrenia: a metaanalysis. Gen Psychiatr 2019; 32: e100091.
42. Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One 2013; 8: e70179.
43. Calarge CA, Mills JA, Ziegler EE, Schlechte JA. Calcium and vitamin D supplementation in boys with risperidoneinduced hyperprolactinemia: a randomized, placebocontrolled pilot study. J Child Adolesc Psychopharmacol 2018; 28:145-50.
44. Cavallaro R, Cocchi F, Angelone SM, Lattuada E, Smeraldi E. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry 2004; 65: 187-90.
45. Tollin R. Use of the dopamine agonists bromocriptine and cabergoline in the management of risperidone-induced hyperprolactinemia in patients with psychotic disorders. J Endocrinol Invest 2000; 23: 765-70.
46. Chanson P, Maiter D. The epidemiology, diagnosis and treatment of Prolactinomas: The old and the new. Best Pract Res Clin Endocrinol Metab 2019; 33: 101290.
47. Melmed S, Kleinberg D. Pituitary masses and tumors. In: Williams Textbook of Endocrinology. Melmed S, Polonsky KS, Reed Larsen P, Kronenberg HM (ed). 13th ed. Philadelphia: Elsevier, 2016, p 232-99.
48. Paepegaey AC, Salenave S, Kamenicky P, et al. Cabergoline tapering Is almost always successful in patients with macroprolactinomas. J Endocr Soc 2017; 1: 221-30.
49. Drake WM, Stiles CE, Bevan JS, et al. A follow-up study of the prevalence of valvular heart abnormalities in hyperprolactinemic patients treated with cabergoline. J Clin Endocrinol Metab 2016; 101: 4189-94.
50. Vroonen L, Lancellotti P, Garcia MT, et al. Prospective, long-term study of the effect of cabergoline on valvular status in patients with prolactinoma and idiopathic hyperprolactinemia. Endocrine 2017; 55: 239-45.
51. Caputo C, Prior D, Inder WJ. The need for annual echocardiography to detect cabergoline associated valvulopathy in patients with prolactinoma: a systematic review and additional clinical data. Lancet Diabetes Endocrinol 2015; 3: 906-13.
52. Steeds RP, Stiles CE, Sharma V, Chambers JB, Lloyd G, Drake W. Echocardiography and monitoring patients receiving dopamine agonist therapy for hyperprolactinaemia: a joint position statement of the British Society of Echocardiography, the British Heart Valve Society and the Society for Endocrinology. Echo Res Pract 2019; 6: G1-G8.
53. Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev 2006; 27: 485-534.
54. Noronha S, Stokes V, Karavitaki N, Grossman A. Treating prolactinomas with dopamine agonists: always worth the gamble? Endocrine 2016; 51: 205-10.
55. Barake M, Klibanski A, Tritos NA. Management of Endocrine Disease: Impulse control disorders in patients with hyperpolactinemia treated with dopamine agonists: how much should we worry? Eur J Endocrinol 2018; 179: R287-96.
56. Athanasoulia-Kaspar AP, Popp KH, Stalla GK. Neuropsychiatric and metabolic aspects of dopaminergic therapy: perspectives from an endocrinologist and a psychiatrist. Endocr Connect 2018; 7: R88-R94.
57. Ioachimescu AG, Fleseriu M, Hoffman AR, Vaughan III TB, Katznelson L. Psychological effects of dopamine agonist treatment in patients with hyperprolactinemia and prolactin secreting adenomas. Eur J Endocrinol 2019; 180: 31-40.
58. Moore TJ, Glenmullen J, Mattison DR. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med 2014; 174: 1930-3.
59. Davie M. Pathological gambling associated with cabergoline therapy in a patient with a pituitary prolactinoma. J Neuropsychiatry Clin Neurosci 2007; 19: 473-4.
60. Dogansen SC, Cikrikcili U, Oruk G, et al. Dopamine agonist- induced impulse control disorders in patients with prolactinoma: a cross- sectional multicenter study. J Clin Endocrinol Metab 2019; 104: 2527-34.
61. De Sousa SM, Chapman IM, Falhammar H, Torpy DJ. Dopa-testotoxicosis: disruptive hypersexuality in hypogonadal men with prolactinomas treated with dopamine agonists. Endocrine 2017; 55: 618-24.
62. Bancos I, Nippoldt TB, Erickson D. Hypersexuality in men with prolactinomas treated with dopamine agonists. Endocrine
2017; 56: 456-7.
63. Bancos I, Nannenga MR, Bostwick JM, Silber MH, Erickson D, Nippoldt TB. Impulse control disorders in patients with dopamine agonist-treated prolactinomas and nonfunctioning pituitary adenomas: a case-control study. Clin Endocrinol (Oxf) 2014; 80: 863-8.
64. Martinkova J, Trejbalova L, Sasikova M, Benetin J, Valkovic P. Impulse control disorders associated with dopaminergic medication in patients with pituitary adenomas. Clin Neuropharmacol 2011; 34: 179-81.
65. Avila A, Cardona X, Bello J, et al. Trastornos del control de los impulsos y punding en la enfermedad de Parkinson: la necesidad de una entrevista estructurada. Neurología 2011; 26: 166-72.
66. De Sousa SMC, Baranoff J, Rushworth RL, et al. Impulse control disorders in dopamine agonist-treated hyperprolactinemia: Prevalence and risk factors. J Clin Endocrinol Metab 2020; 105: 1-11.
67. Burback L. Management of microprolactinoma with aripiprazole in a woman with cabergoline induced mania. Endocrinol Diabetes Metab Case Rep 2015; 2015: 150100.
