EMANUEL J. SAAD 1, 2, PEHUÉN FERNÁNDEZ 2, 3, ANYELO E. CARDOZO AZUA 3, VIRGINIA ELLENA 3, CECILIA DIZ1, GUILLERMO GIORDANO 4, PAMELA BORGOGNO 3, SILVANA NUÑEZ 3, DANIELA SARMANTANO 3, ALEJANDRA GUZMAN 3, FACUNDO SCHWARZ 3, SOFÍA NASER 3, MARÍA FERNANDA FLORES 1, MARÍA LUJÁN ALAYE 3, JUAN PABLO CAEIRO 2, 4, JORGE DE LA FUENTE 2, 4
1 Departamento de Medicina Interna, Hospital Privado Universitario de Córdoba, 2 Instituto Universitario de Ciencias Biomédicas de Córdoba, 3 Departamento de Nefrología, Hospital Privado Universitario de Córdoba, 4 Departamento de Enfermedades Infecciosas, Hospital Privado Universitario de Córdoba, Argentina
Resumen Las infecciones son complicaciones frecuentes de los trasplantes renales. Los objetivos del estudio fueron determinar la frecuencia y el tipo de infecciones que ocurren en el período post-trasplante temprano (0-1 mes), intermedio (1-6 meses) y tardío (6-12 meses) en nuestro medio y analizar los factores de riesgo de infección. Se realizó un estudio de cohorte retrospectivo que incluyó todos los pacientes con trasplantes renales realizados entre 2009 y 2015 en dos hospitales universitarios de tercer nivel de la ciudad de Córdoba, excluidos los receptores de trasplante simultáneo de múltiples órganos sólidos y los menores de 18 años. Fueron incluidos 375 pacientes, de los cuales 235 (62.7%) tuvieron al menos un episodio de infección. Hubo 504 episodios de infección: 131 (26%) ocurrieron en el período temprano, 272 (53.9%) en el intermedio y 101 (20.1%) en el tardío. La mayoría de las infecciones fueron de origen bacteriano (principalmente del tracto urinario). La mayoría de las infecciones virales ocurrieron en el segundo y el tercer período y Citomegalovirus fue el responsable más frecuente. En el análisis multivariado, los factores de riesgo de infección post-transplante renal fueron: edad > 60 años (odds ratio ajustado [aOR] 1.92; IC95% 1.05-3.49), donante cadavérico (aOR 8.19; IC95% 2.32-28.9), uso de catéter pigtail (aOR 4.06; IC95% 1.27-12.9) y número de días internado postrasplante (aOR 1.05; IC95% 1.01-1.11). En conclusión, confirmamos que las infecciones en pacientes con trasplante renal son muy frecuentes en nuestro medio, por lo cual es importante conocer la epidemiología local y los factores de riesgo.
Palabras clave: trasplante de riñón, infecciones urinarias, citomegalovirus, infecciones, inmunosupresión, infecciones oportunistas
Abstract Infections are frequent complications of kidney transplants. We aimed at determining the frequency and type of infections that occur in renal transplant recipients during the early (0-1 month), intermediate (1-6 months) and late (6-12 months) post-transplant period and analyzing the risk factors for infection. To this aim, we conducted a retrospective cohort study on 1-year post-transplant follow-up in two third-level university hospitals in Cordoba city. All consecutive recipients of renal transplants performed between 2009 and 2015 were included, except those with multiple solid organ transplantation and pediatric patients. We included 375 recipients, of which 235 (62.7%) had at least one episode of infection during follow-up. There were 504 episodes of infection, of which 131 (26%) occurred in the early, 272 (53.9%) in the intermediate, and 101 (20.1%) in the late post-transplant period. The most frequent infections in all periods were caused by bacteria (mainly urinary tract infections), and the most frequent viral infection was caused by Cytomegalovirus (mainly in the second and third period). In the multivariate analysis, infection risk factors were: age > 60 years (adjusted odds ratio [aOR] = 1.92; 95% CI = 1.05-3.49), organ transplantation from deceased donor (aOR = 8.19; 95% CI = 2.32-28.9), use of pigtail catheter for urinary tract drainage (aOR = 4.06; 95% CI = 1.27-12.9), and number of days in hospital after transplant (aOR = 1.05; 95% CI = 1.01-1.11). In conclusion, infections in renal transplant recipients represent a very frequent health problem in our hospitals. Understanding the local epidemiology of infection and the potential risk factors for infection acquires utmost importance.
Key words: kidney transplantation, urinary tract infections, cytomegalovirus, infections, immunosuppression, opportunistic infections
Postal address: Emanuel J. Saad, Hospital Privado Universitario de Córdoba, Naciones Unidas 346, Barrio Parque Vélez Sarsfield, 5016 Córdoba, Argentina
e-mail: emanuelsaad@hotmail.com
Current knowledge
• Infections are the most frequent cause of hospital admission in the first year after renal transplantation.
• The risk of infection during the post-transplant period is mainly determined by the nature and intensity of epidemiological exposure, the degree of immunosuppression, and the preventive measures used.
Contribution of the article to current knowledge
• In two third-level university hospitals in Cordoba city, the most frequent infections during the first year posttransplantation affected mainly the urinary tract and were caused by bacteria.
• Infection risk factors were age > 60 years, organ transplantation from a deceased donor, use of pigtail catheter for urinary tract drainage, and number of days in hospital after transplant.
End stage renal disease is an important cause of morbidity and mortality worldwide, and its incidence is growing constantly over the years 1. Although renal transplantation is an effective treatment option with patients experiencing increased long-term survival, the concomitant implementation of immunosuppressive therapies has resulted in an increased risk of developing infections 2, 3. Infections are the main cause of hospital admission within 24 months after renal transplantation and account for prolonged hospital stays and increased healthcare costs. Furthermore, they represent the second leading cause of death in renal transplant recipients after cardiovascular disease 4, 5.
The risk of post-transplant infection is determined by several factors, including the nature and intensity of epidemiological exposure, the degree of immunosuppression, and the preventive measures used 6, 7. The frequency of infectious episodes varies during the first-year posttransplant.
In the first month, infections are mainly caused by nosocomial (hospital-acquired) pathogens, surgical complications, and donor-derived infections. The period between one month and 6 months after transplantation is characterized by maximum immunosuppression, when there is greater susceptibility to develop opportunistic infections.
By contrast, infections developing after 6 months post-transplant are usually caused by microorganisms which are common to the rest of the population 8. It is important to understand the local epidemiology of the leading infectious conditions affecting renal transplant recipients in order to properly manage the patients.
The primary objective of this study was to determine the frequency of infectious disease in renal transplant recipients during a 1-year follow-up period after transplantation in our setting. The secondary objectives were: (i) to characterize the frequency and type of infections that occurred in renal transplant recipients during the early (0-1 month), intermediate (1-6 months), and late (6-12 months) post-transplant periods; (ii) to establish the frequency of reactivation of renal transplant recipient’s infections and opportunistic infections during the post-transplant period; (iii) to determine the frequency of donor-derived infections; and (iv) to assess the risk factors for developing infections during the post-transplant period.
Materials and methods
A retrospective study was carried out in two third-level university hospitals in Córdoba city, Argentina: Hospital Raúl Ángel Ferreyra and Hospital Privado Universitario de Córdoba. All patients aged 18 and older who received a single kidney transplant between January 2009 and December 2015, and who attended their follow-up visits at those hospitals were included. Patients under 18 years of age and those who had received another solid organ transplant during the same period were excluded.
All transplanted patients received immunosuppressive treatment and infection prophylaxis according to the Nephrology Department protocol. Patients with a high immunological risk received anti-thymocyte serum induction therapy (1.5 mg/kg/ day for 5 days), or basiliximab (2 doses of 20 mg) and human gamma globulin (2 doses of 2 g/kg) as an alternative regimen for subjects who were at a higher risk of developing serious infections and cancer. Patients with a high risk of delayed graft function and low immunological risk received basiliximab (same dosage). In addition, all patients received a methylprednisolone pulse therapy for 3 days. Calcineurin inhibitors (preferably tacrolimus), an anti-proliferative agent (preferably mycophenolate), and prednisone were used for maintenance therapy, except for a few special situations. All patients received antibiotic prophylaxis to prevent infection of the surgical site in the immediate post-surgery period, trimethoprim/sulfamethoxazole for one year, valganciclovir for 3 months (subjects who had received anti-thymocyte serum or subjects with negative CMV IgG results before transplant), or acyclovir for an indefinite period (all other patients), and nystatin during the first stage of the transplant. All patients who received an organ from donors with positive serology for syphilis (VDRL test) were given penicillin G benzathine (2 400 000 U). All subjects were tested for CMV, BK virus (BKV) and Chagas’ disease, with a monthly PCR determination up to 6 months. After transplantation, patients attended periodical follow-up controls which enabled the recording of significant events in their medical histories.
The medical history of each patient included in the study was reviewed, and demographics, comorbidities, transplantrelated information, and data about infections developed during the post-transplant period were collected. A distinction was made between standard criteria donors and expanded criteria donors (patients > 59 year or donors aged 50-59 who complied with at least two of the following three criteria: cerebrovascular accident as cause of death, history of hypertension and preablation serum creatinine level > 1.5 mg/dl) 9. Patient follow-up was carried out until one-year post-transplant, until patients underwent a new solid organ transplantation, or until patient death or loss to follow-up, whichever occurred first. The definitions used are found in the supplementary material.
The study was approved by the Hospital ethical review board.
Continuous variables were expressed as mean and standard deviation, and were compared with the Student’s t-test or the Mann- Whitney U test, according to their homogeneity.
Categorical variables were expressed as frequency and percentage, and were compared using the chi-square test or the Fisher’s exact test, according to the expected frequencies.
Statistical significance was defined as a p value < 0.05. Relative risk with 95% confidence interval was used to assess the link between individual risk factors and the development of infections during the post-transplant period. Subsequently, all significant variables with a p value < 0.05 in the univariate analysis were considered for a multivariate logistic regression
analysis. Survival curves of patients who developed infections and patients who did not develop infections were compared, considering the period during which infections developed, using Kaplan-Meier survival curves and the log-rank test.
The analyses were carried out using the Stata 14 statistical software (Stata-Corp. LP., College Station, TX, USA).
Results
Out of 488 patients who received a renal transplant during the study period, 113 (23.1%) were excluded. Of these, 76 (67.3%) were excluded because they had multiple solid organ transplantation, and 37 (32.7%) because they were under 18 years of age. A total of 375 patients were included, of whom 235 (62.7%) had at least one episode of infection during the follow-up period, and 140 had none.
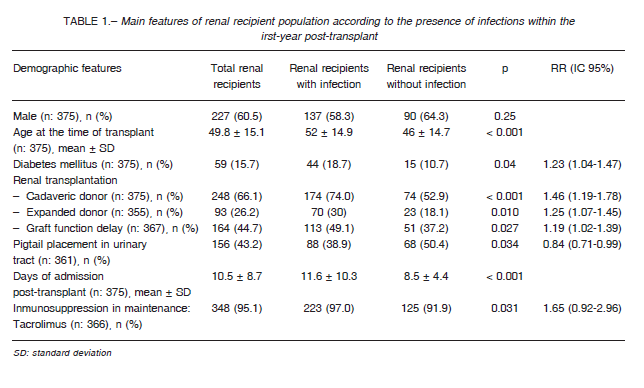
Table 1 summarizes the epidemiological characteristics of the transplanted population together with donor, immunosuppression and transplant-related characteristics.
The patients who had infection episodes were older (52 ± 14.9 vs. 46 ± 14.7 years old; p < 0.001) and had a history of diabetes mellitus more often than those who did not develop infections (18.7 vs. 10.7%; p = 0.04).
Likewise, these patients also had a history of having more frequently received a kidney from a deceased donor (74 vs. 52.9%; p < 0.001) or from an expanded criteria donor (30 vs. 18.1%; p = 0.01), of having a greater delay in graft function (49.1 vs. 37.2%; p = 0.02), less use of pigtail catheter for urinary tract drainage (38.9 vs. 50.4%; p = 0.03), longer hospitalization time after transplant (11.6 ± 10.3 vs. 8.5 ± 4.4 days in hospital; p < 0.001), and more use of tacrolimus as maintenance immunosuppression (97 vs. 91.9%; p = 0.03). All these variables were considered as risk factors for infection after the transplant. The risk of infection increased by 38% in subjects over 60 years (RR = 1.38; 95% CI 1.20-1.60), 23% in diabetic patients (RR = 1.23; 95% CI 1.04-1.47), 46% in patients who received a kidney from a deceased donor (RR = 1.46; CI 95% = 1.19-1.78), 25% in patients who received an organ from an expanded criteria donor (RR = 1.25; 95% CI 1.07-1.45), 19% in patients who experienced a delay in graft function (RR = 1.19; 95% CI 1.02-1.39), and 65% in those patients who received tacrolimus (RR = 1.65; 95% CI 0.92-2.96). There were no significant differences in the presence of comorbidities such as heart failure, ischemic heart disease, chronic obstructive pulmonary disease, neurological, hepatic, rheumatic and neoplastic
diseases between patients who had any episode of infection and those who did not have any during follow-up.
There were also no differences in the modality and time of pre-transplant renal replacement therapy, invasive postsurgical procedures in the urinary tract, episodes of graft rejection, percentage of primary non-functioning transplants and type of immunosuppression used during induction and maintenance (except for tacrolimus). The median follow-up was 12 months and the loss to follow-up was 6.4%, with no differences found between the groups.
In the multivariate analysis, infection risk factors adjusted for all other confounding variables were age > 60 years (adjusted odds ratio [OR] = 1 .92; 95% CI 1.05-3.49), organ transplantation from deceased donors (adjusted OR = 8.19; 95% CI 2.32-28.9), use of pigtail catheter for urinary tract drainage (adjusted OR = 4.06; 95% CI 1.27-12.9), and number of days in hospital after transplant (adjusted OR = 1.05; 95% CI 1.01 -1.11).
Five hundred and four infection episodes were reported, 131 (26%) of which occurred within the first 30 days after transplant, 272 (53.9%) occurred between 31 and 180 days after transplant, and 101 (20.1%) occurred between 181 and 365 days after transplant (Table 2).
According to their etiologic origin, the frequencies were bacterial 350 (69.4%), viral 118 (23.4%), parasitic 21 (4.2%), and fungal 15 (3%). Urinary tract infections (UTIs) were the most frequent infections (44.2%), followed by cytomegalovirus (CMV) infection and disease (23.4%), culture-positive preservation fluid or transplanted organ (6.7%), and surgical site infections (5.2%).
During the early period (0-30 days), most infections were caused by bacteria (87%), and a few infections were caused by viruses (7.6%). The most frequent infections during this period were UTIs (36.6%), culture-positive preservation fluid (26%) and surgical site infections (11.5%). During the intermediate period (31-180 days), there was an increase in the rate of viral infections (33.1%) and a relative decrease in the rate of bacterial infections (57.7%). The most frequent infections during this period were UTIs (40.8%) and CMV infection and disease (31.6%). During the late period (181-365 days), there was an increase in the rate of bacterial infections (78.2%) and a decrease in the rate of viral infections (17.8%). As it was the case in previous periods, UTIs were the most frequent infections (63.4%), followed by CMV infection and disease (13.9%). The least frequent bacterial infections were: Clostridium difficile diarrhea (2 in the early period, 1 in the intermediate and 2 in the late period), septic arthritis (2 in the intermediate period), infectious endocarditis (1 in the early period and 1 in the intermediate period), osteomyelitis (2 in the intermediate period) and tuberculosis (1 in the early period and 1 in the intermediate). Fungal infections were the most infrequent: among them were disseminated candidiasis (1 in the early period) and UTI by Candida spp (1 in the late period).
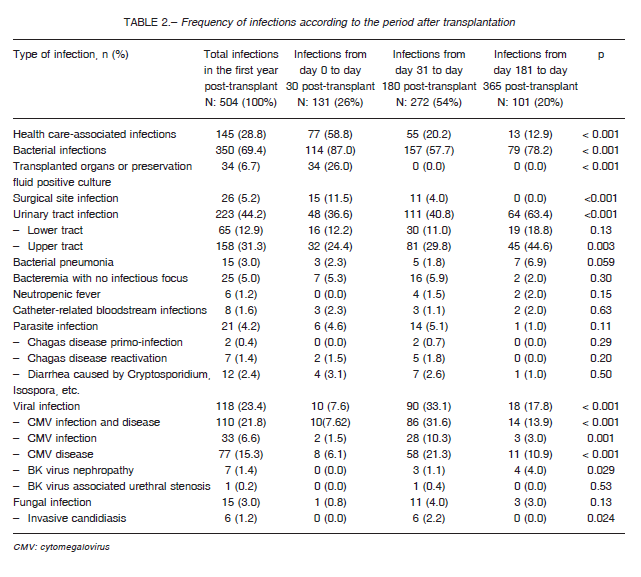
A total of 137 opportunistic infections were identified (27.2% of total infections), 56.2% of which were CMV disease (77 episodes), followed by 33 cases of CMV infection (24.1%), 9 cases of Chagas’ disease (6.6%) and 8 cases of BKV infection (5.8%). In addition, among the opportunistic infections identified, 5 cases of aspergillosis were diagnosed (4 in the intermediate period and 1 in the late period), as were 2 cases of pneumocystosis (1 in the intermediate period and 1 in the late period) and 1 case of nocardiosis (in the late period).
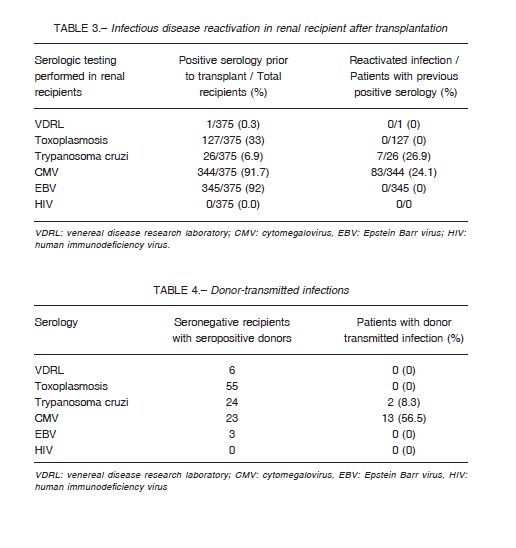
Kidney recipients had pre-transplant serology positive for Epstein Barr virus (EBV) (92%), CMV (91.7%) and Chagas’ disease (6.9%). CMV reactivation was observed in 24.1% of the CMV seropositive patients, and Chagas’ disease reactivation was identified in 26.9% of the patients who tested positive for the disease before transplantation.
No reactivation of other conditions was observed (Table 3).
Among patients with discordant serologies (seronegative recipients with seropositive donors), CMV transmission occurred in 56.5% (13/23) of the patients, and Chagas’ disease transmission occurred in 8.3% (2/24).
Transmission of other donor infections to recipient was not observed (Table 4).
Nineteen patients died (5.1%) during the follow-up period. There were no significant differences in mortality among patients with at least one infection episode and patients who had none (6.4% vs 2.9%; p = 0.13). Likewise, no significant differences were found between the survival curves of both groups at 12-month follow-up (with infection 93.8% [95% CI 89.8-96.3] vs. without infection 97.1% [95% CI 92.3-98.9]; p = 0.14). When analyzing patients’ survival rate after the infection episode, differences in survival curves were found depending on the period in which the infection was diagnosed (early period 88.9% [95%CI 81.5-94.3] vs. intermediate period 96.1% [95%CI 89.9-98.5] vs. late period 100%; p = 0.048). Eleven deaths occurred in patients who had infections in the first period after transplantation (11.1%), 4 occurred among those who developed infections in the second period (3.7%), and no deaths occurred in the third period after transplantation.
Discussion
In our study, 62% of the patients had at least one episode of infection during the first year after transplantation, a rate which is in range with results published by other authors, where the incidence of infection ranged between 40-80% 10-13.
Among the patients included in our study, there were many with a history of previous renal transplant, and the average time on dialysis prior to transplantation was nearly 5 years.
While these elements have been described as risk factors for developing infections, no significant differences were observed between both groups 14. It was only observed that patients who developed infections were older and more frequently diabetic than those who did not. With regard to the transplant, as it has been described in other studies, we found that a history of deceased or expanded criteria donor graft and the delay in graft function was associated with an increased risk of infection 15.
Moreover, we observed that prolonged post-transplant hospitalizations were most frequently associated with the development of infections. Such an increase in the length of post-transplant hospitalization might be due to the occurrence of medical complications, including infections, as a result of an increased length of exposure to nosocomial pathogens and the use of endovascular or urological invasive devices, as it has previously described 10. When comparing the types of immunosuppressive maintenance and induction therapies, we found that the patients who used tacrolimus were at a higher risk of developing infections, as previously reported 16. However, it must be considered that most patients in both groups did use tacrolimus,
which is one of the most widely used immunosuppressive maintenance therapy along with mycophenolate.
In the bivariate analysis, patients who had a pigtail catheter inserted into the urinary tract appeared to be at a lower risk of infection. Actually, this is due to the fact that almost all transplanted patients who received an organ from a living donor (considered as a “protective factor” against infections) had a pigtail catheter, in comparison with a small percentage of patients who received an organ from a deceased donor (considered as a risk factor for infections). When adjusting these variables, the presence of a pigtail catheter was a clear risk factor for developing infections.
When surveying the epidemiology of infections according to the post-transplant period in which they developed, we noted that over half of them occurred between 30 days and 6 months after transplantation, as it has been described in other studies 16, 17. This is a period of maximum immunosuppression, and thus, it is reasonable to find it associated to a higher risk of developing opportunistic infections 16.
In our study, the most frequent cause of infection in this period was CMV and, to a lesser extent, Trypanosoma cruzi (agent of Chagas disease) and Pneumocystis.
However, it should be noted that most bacterial infections during the first year after transplantation occurred in this period (pyelonephritis had the highest frequency).
Several studies indicated that bacterial infections are the most frequent in transplant patients, accounting for 50-70% of the episodes10, 18. When these infections include bacteremia, mortality rate can be as high as 50% mortality.
The main risk factors promoting their development are the use of urinary catheters, intravascular catheters, surgical procedures, history of CMV infection, and episodes of rejection 19, 20. As observed in our study, urinary infections have been reported as the main cause of bacterial infection in patients with renal transplantation 21, 22.
According to the literature, the lowest number of infection episodes during the first year post-transplant occur in the first 30 days after transplantation8, 16. In our study, however, it was during the third period post-transplant when the lower number of infection events occurred, with a slight difference in frequency with respect to the first period. As previously reported, most infections occurring in the first month were of nosocomial origin, mainly secondary to donor-derived infections, surgical site infections, UTI and endovascular catheter-related infections 10, 17.
It has also been described that infections occurring after 6 months post-transplant tend to be similar to the ones developed by the rest of the population in general.
This could be due to the fact that the period of highest immunosuppression, as well as the transplant-related risks factors, have been overcome 8. In our study, the vast majority of infections occurring during this third period were community-acquired bacterial infections, mainly urinary infections and bacterial pneumonias. However, graft rejection episodes may increase the risk of opportunistic infections, mostly due to the need to increase the length of immunosuppression 8, 17. Opportunistic infections are of great relevance since their clinical manifestations tend to be atypical in transplant patients, and there may be delays in diagnosis and appropriate treatment 23. In this study, about one-quarter of infections were secondary to opportunistic microorganisms, and they occurred between 30-180 days after transplantation. Many of them resulted from the reactivation of the recipient’s dormant viral infections, as well as from donor-transmitted infections. This demonstrates the importance of a complete pre-transplant screening of donor and recipient for the prevention and early detection of these kind of infections 15, 24.
Just like it is mentioned in the literature, CMV was the main opportunistic agent identified in this study 25, 26. CMV reactivation can develop as an infection (with a mere increase in the number of viral copies) or as a disease (with associated symptoms such as fever, general distress, leukopenia, thrombocytopenia or evidence of tissue invasion) 26. As it has been noted by other authors, the prevalence of CMV positive serology was very high among kidney donors and recipients 25. This a relevant aspect, since the greatest known risk factor for CMV disease is a mismatch between a CMV seropositive donor and a CMV seronegative recipient before transplantation 27. In a study from Argentina, CMV accounted for 50% of the diarrhea episodes with identified microbiological agent in patients with kidney and simultaneous pancreas-kidney transplantation, being diarrhea one of the main manifestations of this infection 28.
Chagas’ disease is an endemic zoonotic disease in several Latin American countries. It is estimated that in Argentina there are 2.5 million infected people 29, 30. There are some reports about the rate of reactivation and transmission during the post-transplantation period 31-33. Riarte et al reported that, in a transplantation center in Argentina, 21% of renal transplant recipients with positive serology tests for Chagas experienced disease reactivation between 1989 and 1996 34. This percentage is very similar to the one observed in our study. On the other hand, those authors reported that donor transmission was close to 18%, in comparison with the 8% transmission rate observed in our study. This infection became of utmost importance since in the last decades it has spread to other continents via various means of transmission, such as vertical transmission, blood transfusion, and organ donation 15, 35, 36.
Another important infection for renal transplant recipients is the one caused by BKV, which can be associated to a wide range of clinical manifestations, such as asymptomatic viruria, urethral stricture, interstitial nephritis, and graft nephropathy 17, 24, 37. As in most studies, we found the frequency of BKV infection to be higher after the first post-transplant month, and mainly manifested as BKV nephropathy, albeit in a much lower percentage (1.4%) compared to reported rates (2-5%) 16. In a study carried out in Argentina, with serial search in kidney transplant recipients, 12% of post-transplant patients were found to be infected with BKV 38.
Tuberculosis is another infection that can develop in immunosuppressed patients. In Argentina, its incidence was 23.9 per 100 000 people in 2016, signaling an upward trend compared to previous years. In our study, there were two cases of tuberculosis, both of which occurred within 6 months post-transplant. While tuberculosis may be donor-transmitted or community-acquired, it usually develops as a reactivation of a latent infection in the recipient 17, 39. In one of the patients who developed tuberculosis, the result of the tuberculin skin test prior to transplantation was not recorded and in the other it was recorded as being negative. This test often gives false-negative results in anergic patients, such as those affected by chronic kidney diseases 39. Thus, it is still possible that this transplant recipient had reactivated a latent tuberculosis infection. A large Argentine study conducted between the 1980s and 1990s reported 3.6% of tuberculosis in kidney transplant recipients with an average time of diagnosis at 13 months 40.
Similarly to findings of other studies, infection episodes caused by fungi accounted for a small percentage, with Candida sp. as the main causative agent 41. Another fungal agent isolated was Pneumocystis jiroveci, which accounts for severe pulmonary infections in transplant patients during the first 3-6 months after transplantation. However, the incidence of this infection has dropped substantially due to the use of antimicrobial prophylaxis with trimethoprim/sulfamethoxazole 23.
It should be emphasized that in our study we observed no case of toxoplasmosis, cryptococcosis, mucormycosis, histoplasmosis or EBV-related infections.
We conclude that infections in renal transplant recipients represent a very frequent and important problem in our setting. Thus, understanding the local epidemiology of infections and their potential risk factors is of special significance in order to design and implement appropriate measures for their prevention and/or timely treatment.
Acknowledgments: We thank Mailen Konicoff, Carla Zaninetti and Claudio Abiega for the technical support provided and Fundación Nefrológica de Córdoba for the economic support for publication costs.
Conflicts of interest: None to declare
References
1. Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl 2015; 5: 2-7.
2. Douthat WG, Fernández P, Rechene J, et al. Trasplante renal y disminución de la mortalidad en los programas de diálisis crónica. Medicina (B Aires) 2014; 74: 1-8.
3. Kritikos A, Manuel O. Bloodstream infections after solidorgan transplantation. Virulence 2016; 7: 329-40.
4. Hamandi B, Husain S, Humar A, Papadimitropoulos EA. Impact of infectious disease consultation on the clinical and economic outcomes of solid organ transplant recipients admitted for infectious complications. Clin Infect Dis 2014; 59: 1074-82.
5. de Carvalho MA, Freitas FGR, Junior HTS, Bafi AT, Machado FR, Pestana JOM. Mortality predictors in renal transplant recipients with severe sepsis and septic shock. PLoS One 2014; 9: e111610.
6. Green M. Introduction: Infections in solid organ transplantation. Am J Transplant 2013; 13 Suppl 4: 3-8.
7. Fishman JA, Issa NC. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect Dis Clin North Am 2010; 24: 273-83.
8. Fishman JA. From the classic concepts to modern practice. Clinical microbiology and infection. Clin Microbiol Infect 2014; 20 Suppl 7: 4-9.
9. Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis 2008; 52: 553-86.
10. Ak O, Yildirim M, Kucuk HF, Gencer S, Demir T. Infections in renal transplant patients: risk factors and infectious agents. Transplant Proc 2013; 45: 944-8.
11. Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant 2006; 20: 401-9.
12. Sousa SR, Galante NZ, Barbosa DA, Pestana JO. [Incidence of infectious complications and their risk factors in the first year after renal transplantation]. J Bras Nefrol 2010; 32: 75-82.
13. Freuler C, García Posadas M, Sanchez AV, et al. Trying to understand infections in transplant patients in a private hospital in Buenos Aires, Argentina. Int J Infect Dis 2016; 45: 196.
14. Shao M, Wan Q, Xie W, Ye Q. Bloodstream infections among solid organ transplant recipients: epidemiology, microbiology, associated risk factors for morbility and mortality. Transplant Rev (Orlando) 2014; 28: 176-81.
15. Len O, Pahissa A. Infecciones transmitidas por el donante. Enferm Infecc Microbiol Clin 2007; 25: 204-12.
16. Ramírez-Zermeño AE, Marcial-Guzmán M, Torres- González MA, Cerrillos-Gutiérrez JI, Rojas-Campos E, Gómez-Navarro B. Infecciones en trasplantados renales en Centro Médico Nacional de Occidente. Rev Mex Traspl 2016; 5: 102-12.
17. Kaul A, Chauhan TS. Opportunistic infection in renal transplant recipients. Indian J Transplant 2014; 8: S57-S64.
18. Medina JC, Antelo V, Nin M, et al. Infecciones bacterianas en pacientes receptores de trasplante renal y
reno-páncreas: alta incidencia de microorganismos multirresistentes. Rev Med Urug (Montev) 2012; 28: 190-8.
19. Camargo LF, Marra AR, Pignatari AC, et al. Nosocomial bloodstream infections in a nationwide study: comparison between solid organ transplant patients and the general population. Transpl Infect Dis 2015; 17: 308-13.
20. Berenger BM, Doucette K, Smith SW. Epidemiology and risk factors for nosocomial bloodstream infections in solid organ transplants over a 10-year period. Transpl Infect Dis 2016; 18: 183-90.
21. Pinheiro HS, Mituiassu AM, Carminatti M, Braga AM, Bastos MG. Urinary tract infection caused by extended- spectrum beta-lactamase-producing bacteria in kidney transplant patients. Transplant Proc 2010; 42: 486-7.
22. Al Midani A, Elands S, Collier S, Harber M, Shendi AM. Impact of urinary tract infections in kidney transplant recipients: a 4-year single-center experience. Transplant Proc 2018; 50: 3351-5.
23. López Oliva M CJ, de la Torre Cisneros J, del Castillo Domingo. Infecciones en el Trasplante Renal. In: Lorenzo V LGJ, ed. Nefrología al Día 2018.
24. Cukuranovic J, Ugrenovic S, Jovanovic I, Visnjic M, Stefanovic V. Viral infection in renal transplant recipients. Scientific World Journal 2012; 2012: 820621.
25. Arias-Murillo YR, Osorio-Arango K, Cortés JA, Beltrán M. Seroprevalencia de citomegalovirus en donantes de órganos y receptores de trasplante renal, Colombia, 2010-2014. Biomédica 2016; 36: 187-93.
26. Contreras K, Vargas MJ, Manrique J, García PK, Rodríguez MP, González CA. Incidencia y características clínicas de pacientes trasplantados renales con infección y enfermedad por citomegalovirus en un centro de trasplante. Acta Med Colomb 2018; 43: 21.
27. Díaz-Betancur J, Henao JE, Jaimes FA. Effects of cytomegalovirus infection and disease in renal transplant recipients. Acta Med Colomb 2012; 37: 131-7.
28. Carena AA, Boughen S, Gagliardi MI, Galante M. Diarrrea aguda en trasplantes renales y reno-pancreas. Medicina (B Aires) 2015; 75: 29-36.
29. World Health Organization. Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis: World Health Organization; 2012.
30. Jörg M, Storino R. Consenso de la Enfermedad de Chagas: La enfermedad de Chagas en el siglo XXI. Consenso para una asignatura pendiente. Rev Argent Cardiol 2002; 70: 9-10.
31. Ortiz AM, Troncoso P, Sainz M, Vilches S. Prophylaxis and treatment of chagas disease in renal transplant donor and recipient: Case report. Transplant Proc 2010; 42: 393-4.
32. Cicora F, Escurra V, Bibolini J, Petroni J, González I, Roberti J. Cerebral Trypanosomiasis in a renal transplant recipient. Transpl Infect Dis 2014; 16: 813-7.
33. Cicora F, Escurra V, Silguero S, González IM, Roberti JE. Use of kidneys from Trypanosoma cruzi infected donors in naive transplant recipients without prophylactic therapy: The experience in a high-risk area. Transplantation 2014; 97: e3-4.
34. Riarte A, Luna C, Sabatiello R, et al. Chagas’ disease in patients with kidney transplants: 7 years of experience 1989-1996. Clin Infect Dis 1999; 29: 561-7.
35. Bern C. Chagas’ Disease. N Engl J Med 2015; 373: 456-66.
36. Lattes R, Lasala MB. Chagas Disease in the immunosuppressed patient. Clin Microbiol Infect 2014; 20: 300-9.
37. Echeverría M, Basilotta N, Aguiar A, et al. Neuropatía por virus BK post trasplante renal diagnóstico y seguimiento por PCR en tiempo real. Medicina (B Aires) 2007; 67: 719-22.
38. Cobos M, Aquila L, Garay E, et al. Epidemiologic study and genotyping of BK Virus in renal transplant recipients. Transplant Proc 2018; 50: 458-60.
39. Meije Y, Piersimoni C, Torre-Cisneros J, Dilektasli AG, Aguado JM, Hosts ESGoIiC. Mycobacterial infections in solid organ transplant recipients. Clin Microbiol Infect 2014; 20 Suppl 7: 89-101.
40. Lattes R, Radisic M, Rial M, Argento J, Casadei D. Tuberculosis in renal transplant recipients. Transpl Infect Dis 1999; 1: 98-104.
41. Badiee P, Alborzi A. Invasive fungal infections in renal transplant recipients. Exp Clin Transplant 2011; 9: 355-62.
Supplementary material
Definitions
– Transplant patient: A patient who has received a renal graft from a living or deceased donor and is under immunosuppressive therapy.
– Delayed graft function (DGF) after renal transplantation: The need for dialysis within the first week after transplantation1.
– Renal allograft rejection: Acute deterioration of graft function associated with specific histological changes2.
Infections
Health care-associated infections (HCAIs): Infections that developed at least 48 hours after hospital admission and for which there was no evidence at the time of admission3.
Urinary tract infection (UTI): They are classified as: A) Lower UTI: Clinically significant bacteriuria (> 105 CFU/ml, or > 102 CFU/ml in urine sample collected after catheter insertion), in association with symptoms of dysuria without tenderness or pain in the proximity of the transplanted kidney, with or without deterioration of graft function; B) Upper UTI: Clinically significant bacteriuria (> 105 CFU/ml, or >102 CFU/ml in urine sample collected after catheter insertion), temperature > 38 °C and/or tenderness or pain in the proximity of the graft, and/or deterioration of renal function, blood tests showing high levels of inflammatory markers (C-reactive protein or leukocytosis) and/or renal image or biopsy compatible with pyelonephritis4.
Bacterial pneumonia: New infiltrates on chest X-ray or computed tomography, along with three of the following:
A) body temperature above 38 °C or below 36.5 °C, B) pathologic sounds to auscultation (crackles, rales, hypoventilation,
tubal murmur, etc.), C) leukocytosis or leukopenia (> 10 000 cells/ml or < 3 000 cells/ml), D) positive sputum culture or purulent sputum (> 25 leukocytes per field and an epithelial cell count <10) secondary to bacterial infection other than mycobacteria5.
Pulmonary tuberculosis: Presence of clinical features and radiographic imaging compatible with tuberculosis, along with a method to confirm the presence of Mycobacterium tuberculosis (acid-fast bacilli stain, Lowenstein-Jensen mycobacterial culture or positive PCR for M. tuberculosis)6.
Bacteremia: True positive blood culture is defined as the growth of one or more microorganisms in at least one sample of blood culture, except for those microorganisms that are potential skin contaminants (such as Diphteroides, Propionibacterium, Bacillus, coagulase-negative Staphylococcus, Corynebacterium and Streptococcus viridans), in which case they meet one of the following criteria: A) Development in two or more blood culture samples; B) Isolation of the same microorganism in another site considered as the source of bacteremia; C) Intravascular infection, together with body temperature above 38 °C or below 36 °C without another pathogen isolation, which motivates the start of a specific antimicrobial therapy against it. All blood cultures associated to the same isolation, with the same antimicrobial sensitivity and focus of infection are considered as one episode that occurs in the same person, during the same period of time. All those episodes in which two or more species of microorganisms are isolated in one or more blood cultures obtained at the same moment are considered as polymicrobial blood cultures, according to the definition of true positive blood culture. Those cases of true bacteremia in which the identification of the probable focus of infection is not possible are registered as bacteremia with no infectious focus7.
Catheter-associated infection: It is defined according to the records in the medical history, using any of the following two criteria: A) Catheter tip culture of > 15 CFU/ml associated to blood culture with isolation of the same microorganism, plus one of the following: 1) Fever > 38 °C, 2) local pain, 3) erythema, 4) warm; B) Blood culture from the catheter that becomes positive at least 2 hours earlier than the peripheral blood culture, with isolation of the same microorganism in both cultures8.
Surgical site infection: An infection that occurs in or near the surgical site within 30 days after surgery (in cases of deep surgical procedure, the period is extended past 90 days), along with purulent discharge from the site or isolation of the same microorganism in blood culture or fluid draining from the surgical site9.
Clostridium difficile-associated diarrhea: Presence of diarrhea, plus one of the following: A) C. difficile toxin detected by enzyme linked immunosorbent assay (ELISA) in culture-positive stool specimen; B) Identification of C. difficile by stool PCR assay10.
Osteomyelitis: It must meet one of the following criteria: A) Positive bone culture; B) Pathologic anatomy compatible with osteomyelitis; C) Positive blood culture, along with diagnostic imaging test findings compatible with osteomyelitis, and no other probable focus of infection11.
Citomegalovirus (CMV) infection: Evidence of virus replication regardless of symptoms. Although there is not a minimum preset value of viral load for considering the replication of CMV as significant, the presence of more than 5 000 copies or the increase in the number of copies by more than 1 000 in one week is considered as a reference value12.
CMV disease: Evidence of virus replication along with symptoms such as fever, general distress, leukopenia, and thrombocytopenia, or evidence of tissue invasion (pneumonitis, hepatitis, gastrointestinal tract disease), which is defined as a positive qualitative PCR (Q-PCR) test result for tissue sample (bronchoalveolar lavage, liver biopsy, bowel biopsy, etc.)12.
Polyomavirus BK (BKV) infection: The infection caused by this virus is related to the following pathologies:
A) BKV-associated nephropathy: Renal function deterioration and presence of positive viral load by PCR in plasma (positive viral load in plasma (> 4 log10) or urine (positive viral load in urine > 7 log10), or positive Q-PCR in renal biopsy tissue or immunohistochemistry identification in renal biopsy; B) BKV-associated hemorrhagic cystitis: Signs and symptoms of hemorrhagic cystitis (macroscopic and microscopic hematuria associated to dysuria or pollakiuria) along with positive viral load by PCR in plasma or urine; C) Ureteral stenosis: Ureteral stenosis diagnosed thorough imaging, along with positive urine and/or plasma BKV viral load12.
Chagas’ disease: Infection caused by the protozoan parasite Trypanosoma cruzii. Its manifestation can be:
A) Primary infection: Presence of positive serology (2 or more methods: indirect hemagglutination test, indirect immunofluorescence assay, enzyme like immunosorbent assay) in a patient with negative pre-transplant serology and/or plasma parasitic load > 50 copies/ml, or positive hemoparasitological exam; B) Reactivation: Recipient with positive pre-transplant cytology (2 or more methods), who presented a plasma parasitic load > 50 copies/ml or positive blood parasitology after transplantation13.
Toxoplasmosis: The infection caused by Toxoplasma gondii in solid organ transplant recipients can be the result of primary infection (seronegative patient before transplantation who receives an organ from a seropositive donor) o reactivation of dormant infection (patient with positive pre-transplant serology who develops the infection). In both cases, the infection is defined as: A) Signs and symptoms compatible with brain abscess, chorioretinitis, systemic disease (fever, adenopathy, general distress), or pneumonitis, plus B) seroconversion, demonstration through tachyzoites biopsy or positive PCR in the affected tissue13.
Cryptosporidium/ Isospora belli/ Cyclospora/ Microsporidium/ Blastocystis hominis/ Giardia-associated diarrhea: Presence of compatible clinical manifestations (usually chronic diarrhea), associated with observation of parasites or parasite eggs in stool examination (stool ova and parasites test)13.
Candidiasis: Infections caused by Candida sp. are classified as: A) Candidemia: Presence of fungus in at least one blood culture sample, in the absence of demonstrable visceral involvement, except for the cutaneous-mucosal location; B) Localized deep-seated candidiasis: Visceral involvement that affects a single organ, requiring histopathological demonstration of the presence of Candida sp. in the tissues involved studies; C) Disseminated candidiasis: infection located in multiple visceral organs which can be demonstrated by means of biopsy or autopsy, or persistent candidemia located in a single visceral organ; D) Urinary tract candidiasis: Presence of at least 3 of the following symptoms: dysuria, pollakiuria, urinary urgency and suprapubic pain (lower UTI), or iliac fossa pain or fever (upper UTI), along with positive urine culture for Candida sp. of >103 CFU/ml. In asymptomatic patients, UTI is considered when 2 cultures of > 105 CFU/ml are recorded14.
Pneumocystosis: Presence of compatible signs and symptoms (fever, dyspnea, non-productive cough, asthenia) which may or may not be accompanied by compatible findings in imaging tests (bilateral, interstitial and diffuse infiltrates) and are associated with one of the following criteria: A) Positive PCR for Pneumocystis jiroveci in sputum, bronchoalveolar lavage or lung biopsy samples; B) Positive methenamine-silver staining in sputum, bronchoalveolar lavage or lung biopsy; C) Positive immunofluorescent staining in sputum, bronchoalveolar lavage or lung biopsy15.
Nocardiosis: Presence of microbiological isolation by PCR, anatomic pathology specimen staining or culture, in association with compatible clinical form of pulmonary, central nervous system or systemic condition, or a condition affecting unusual sites (skin, bones, kidneys, joints, etc.)16.
Aspergillosis: Identification of the microorganism by blood culture, tissue culture, positive PCR or galactomannan antigen detection test, along with compatible clinical manifestations17.
Infective endocarditis (IE): Defined on the basis of the modified Duke criteria for IE18.
Septic arthritis: Presence of compatible clinical manifestations (joint pain, functional limitation and fever > 38.5 °C) and positive synovial fluid culture with inflammatory findings19.
Neutropenic fever: Presence of fever > 38°C, sustained for 1 hour, associated to neutrophil count of less than 500 cells/mm3 or a neutrophil count that is expected to fall below 500 cells/mm3 within the next 48 hours20.
References
1. Denecke C, Biebl M, Fritz J, et al. Reduction of cold ischemia time and anastomosis time correlates with lower delayed graft function rates following transplantation of marginal kidneys. Ann Transplant 201 6; 21:246-55.
2. Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014; 14: 272-83.
3. Bennett JV, Jarvis WR, Brachman PS. Bennett & Brachman’s hospital infections. Philadelphia, USA: Lippincott Williams & Wilkins, 2007.
4. Gozdowska J, Czerwinska M, Chabros L, et al. Urinary tract infections in kidney transplant recipients hospitalized at a transplantation and nephrology ward: 1-year follow-up. Transplant Proc 2016; 48: 1580-9.
5. Qin Q, Shen KL. Community-acquired pneumonia and its complications. Indian J Pediatr 2015; 82: 745-51.
6. CDC. Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morbidity and mortality weekly report 2009; 58: 7.
7. Saad EJ, Baenas DF, Boisseau CS, et al. Características de las infecciones del torrente sanguíneo en pacientes adultos de dos centros de tercer nivel de córdoba, argentina. Rev Fac Cien Med Univ Nac Cordoba 2018; 75: 156-67.
8. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49: 1-45.
9. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309-32.
10. Biel M, Javier F. Diarrea por Clostridium difficile. Gastroenterol Latinoam 2010; 21: 260-7.
11. CDC. CDC/NHSN surveillance definitions for specific types of infections. CDC, Atlanta 2014: 1-24.
12. Karuthu S, Blumberg EA. Common infections in kidney transplant recipients. Clin J Am Soc Nephrol 2012; 7: 2058-70.
13. Kotton C, Lattes R, Practice AIDCo. Parasitic infections in solid organ transplant recipients. Am J Transplant 2009; 9: S234-S51.
14. Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009: 503-35.
15. Eitner F, Hauser IA, Rettkowski O, et al. Risk factors for Pneumocystis jiroveci pneumonia (PcP) in renal transplant recipients. Nephrol Dial Transplant 2010; 26: 2013-7.
16. Yu S, Wang J, Fang Q, Zhang J, Yan F. Specific clinical manifestations of Nocardia: A case report and literature review. Exp Ther Med 2016; 12: 2021-6.
17. Patterson TF, Thompson III GR, Denning DW, et al. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America Clin Infect Dis 2016; 63: 433-42.
18. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132: 1435-86.
19. Ornelas-Aguirre JM. Septic arthritis in adults in a tertiary care center. Reumatol Clin 2016; 12: 27-33.
20. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52: e56-e93.
