IVÁN HUESPE 1, 2, EDUARDO PRADO 1, INÉS STANELONI 3, NICOLÁS CONTRERA 1, LISANDRO DENADAY 4, EDUARDO SAN ROMAN 1, JORGE SINNER 1
1 Terapia Intensiva de Adultos, Hospital Italiano de Buenos Aires, 2 Instituto de Medicina Traslacional e Ingeniería Biomédica HIBA-IUHI-CONICET, 3 Servicio de Infectología, Hospital Italiano de Buenos Aires, 4 Laboratorio central, Hospital Italiano de Buenos Aires, Argentina
Resumen La cinética de la procalcitonina es útil para reducir la duración de la antibioticoterapia en pacientes críticos, pero no se analizó su rol en infecciones por gérmenes multirresistentes. Se realizó un estudio observacional retrospectivo, analizando las curvas de procalcitonina de pacientes con neumonías asociadas a ventilación mecánica (NAVM) y bacteriemias asociadas a catéter (BAC) con rescate bacteriano durante el período 1/11/16 a 1/7/19. Se estudiaron 16 pacientes con infección por gérmenes sensibles (10 BAC y 6 NAVM) y 10 por gérmenes multirresistentes (10 BAC y 10 NAVM). Los pacientes con BAC generadas por gérmenes multirresistentes presentaron valores de procalcitonina mayores que los pacientes con BAC por gérmenes sensibles: (39 ± 30 μg/l vs. 10.7 ± 11 μg/l, p = 0.02). Los pacientes con NAVM generada por gérmenes sensibles y multirresistentes presentaron valores de procalcitonina similares. El descenso de procalcitonina a niveles 80% menores al valor máximo o menores a 0.5 μg/l (con tratamiento antibiótico efectivo) fue más veloz en pacientes con infección por gérmenes sensibles (5 ± 1.8 días vs. 7.2 ± 2.9 días, p = 0.03). En las infecciones por gérmenes multirresistentes, la respuesta inflamatoria medida por procalcitonina fue más intensa y prolongada, aun con un tratamiento antibiótico efectivo. Sin embargo, el descenso se produjo antes de que finalizaran los esquemas antibióticos convencionales. Por este motivo, se considera necesario estudiar la potencial utilidad de protocolos antibióticos guiados por procalcitonina en pacientes con infecciones por gérmenes multirresistentes para reducir la exposición a antibióticos.
Palabras clave: procalcitonina, bacterias multirresistentes, resistencia a carbapenems, inflamación
Abstract Procalcitonin guidance stimulates a reduction in the duration of antibiotic treatment in critically ill patients with a presumed bacterial infection, but its role in infections caused by multidrug-resistant bacteria has not been sufficiently explored. In this retrospective observational study, we analyzed procalcitonin curves of 32 patients with culture-confirmed ventilation-associated pneumonia (VAP) and catheter-related bloodstream infections (CRBSI) occurred during the period 11/1/2016 to 7/1/2019. Sixteen infections were caused by multidrug-resistant bacteria (10 CRBSI and 6 VAP) and other 16 by sensitive bacteria (10 CRBSI and 6 VAP). CRBSI generated by multidrug-resistant bacteria elicited significantly higher procalcitonin levels than CRBSI infections caused by sensitive bacteria (39 ± 30 μg/l vs. 10.7 ± 11 μg/l, p = 0.02). Patients with VAP caused by sensitive and multidrug-resistant bacteria elicited similar procalcitonin levels. The time to a decrease in procalcitonin level to less than 80% of the peak value or less than 0.5 μg/l upon effective antibiotic treatment was 7.2 ± 2.9 days in multidrug-resistant bacteria vs. 5 ± 1.8 days in sensitive bacteria (p = 0.03). In multidrug-resistant bacteria, the inflammatory response measured by procalcitonin is stronger and longer, even with an effective antibiotic treatment. However, the decline occurs before the conventional antibiotic scheme is completed. The potential application of antibiotic protocols guided by procalcitonin to these groups of patients grants further studies aimed to reduce exposure to antibiotics in critical multidrug-resistant infections.
Key words: procalcitonin, multidrug-resistant bacteria, carbapenem resistance, inflammation
Postal address: Iván Huespe, Hospital Italiano de Buenos Aires, Tte. Gral. Juan Domingo Perón 4190, 1199 Buenos Aires, Argentina
e-mail: ivan.huespe@hospitalitaliano.org.ar
Current knowledge
• Procalcitonin kinetics in septic patients at intensive care units is well known. However, procalcitonin levels and dynamics in critically ill patients suffering from infections due to multidrug-resistant bacteria have not been studied.
In fact, several publications excluded this population from their analysis.
Article´s contribution to knowledge
• We compared procalcitonin levels and clearance rates induced by sensitive and multidrug-resistant bacteria in patients with catheter-related bloodstream infections and ventilation-associated pneumonia. We found higher procalcitonin levels and a slower descent in multidrug-resistant infections, suggesting that this kind of infection elicits stronger inflammatory responses. As the decline of PCT levels occurred well before the completion of antibiotic treatment, PCT guidance could help to shorten antibiotic treatment courses of multidrug-resistant infections.
Multidrug-resistant (MDR) bacteria are selected when the patient takes antibiotics in such a way that antibioticsensitive bacteria are overgrown by resistant clones 1. The longer the exposure to antibiotics the higher the pressure to select for antibiotic resistance 2-4. Nowadays, the clinical threat comes mainly from species such as Escherichia coli and the group known as the ESKAPE organisms (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter spp, Pseudomonas spp, Enterobacter spp), which are found harmlessly in us, on us, or in our environment 4, 5.
Up to 30-50% of the antibiotics administered to hospitalized patients may be unnecessary 5, 6, and the courses of treatment usually exceed the recommended duration 7.
This is particularly critic in intensive care units (ICU) where broad-spectrum antibiotic schemes are empirically administered and, in most cases, it is not possible to de-escalate the therapy because the pathogen remains unidentified in 40 to 60% of septic patients 8, 9.
Therefore, it is interesting to analyse the new approaches to reduce exposure through a selective starting of antibiotics and shortening the duration of treatments 10, 4.
In this sense, biomarkers are being investigated to facilitate the diagnosis of infection, to initiate early antimicrobial treatment, and to help in decision-making regarding its termination, to avoid unnecessarily long antibiotic therapies.
Procalcitonin (PCT) is the blood analyte most studied as a biomarker of bacterial infection as well as for monitoring and ending antibiotic therapy 11. Multiple randomized clinical trials (RCTs) have evaluated the usefulness of PCT to guide the initiation and completion of antibiotic therapy. The results of these studies show that PCT guidance stimulates a reduction in treatment duration and daily defined doses of antibiotics in critically ill patients with presumed bacterial infections, stopping prescribed antibiotics if procalcitonin concentration had decreased by 80% or more of its peak value, or when it reached a value of 0.5 μg/l or lower. However, the large RCTs that evaluated PTC-guided completion of antibiotic courses included mostly patients with community infections. This is evidenced by the low rate of MDR infections included.
For example, MDR infections represented only 17% of the cases in the PRORATA trial and they were not analyzed separately. In fact, most large RCTs were not planned to evaluate infections by MDR bacteria 12-15.
Patients with MDR infections might not respond in a conventional way to antibiotic treatments and we do not know if those protocols can be applied to them. Therefore, our aim was to investigate PCT kinetics and the time necessary for a decrease to less than 80% of the peak value or below 0.5 μg/l in patients with MDR infections compared to values observed in patients infected with sensitive bacteria (SB). Because ventilation associated pneumonia (VAP) and catheter-related bloodstream infections (CRBSI) are the infections with the highest incidence of MDR infections (30%-50%) 16, we studied the kinetics of PCT exclusively in these population groups, in order to obtain similar numbers of MDR and SB infection episodes 17, 18.
Materials and methods
A retrospective observational study was carried out in a 38-bed surgical and medical ICU of our high complexity hospital. All patients with bacterial isolates of VAP and CRBSI during the period from 11/1/16 to 7/1/19 were analyzed. Patients who presented daily measurements of PCT with values higher than 0.5 μg/l secondary to VAP or CRBSI were selected. Measurements were followed until their decrease to values ≤ 0.5 μg/l or lower than 80% of the maximum value. Only the first isolate of VAP or CRBSI was analyzed per patient.
Patients were excluded in the following events: an infectious intercurrence during the antibiotic course, death before the expected decrease in PCT levels, chronic infections due to undrained abscesses, endocarditis, tuberculosis or meningitis, and patients already with antibiotics considered effective for the bacteria isolated at the time of bacteriological sampling.
The MDR bacteria evaluated were those considered as a priority by the World Health Organization 19: Carbapenemresistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus sp.
The serum PCT levels were measured by the VIDAS BRAHMS PCT assay using the bioMérieux VIDAS analyzer (coefficient of variation 3.8%). PCT plasma concentrations were expressed in μg/l, and they ranged from 0.05 μg/l to 200 μg/l. PCT values were evaluated at the time of bacteriological isolation and on consecutive days until the PCT value fell below 0.5 μg/l or decreased 80% of the maximum value reached. The antibiotic schemes started empirically, and the schemes indicated once the specific bacterium was detected, were registered.
The presence of acute renal failure was evaluated with the Acute Kidney Injury Network (AKIN) classification 20, the urinary output, and the serum creatinine concentrations were assessed in the peak of PCT. Creatinine concentrations were compared with baseline concentrations; the baseline creatinine was considered the value before admission (if it had been determined less than 3 months before) or the first value after hospital admission. Creatinine concentrations were measured using the compensated kinetic Jaffé method (coefficient of variation: 3.4%).
The Charlson and SOFA scores were evaluated in the ICU admission, the days of permanence in ICU and hospital were also registered.
Statistical analyses were performed with the program “STATA 13”. The distribution of numerical results was calculated using the Kolmogorov-Smirnoff normality test. Normally distributed data were presented as mean ± standard deviation (SD) and the comparisons between two groups were carried out with the two-sample t-test. Data following a non normal distribution were presented as median and interquartile range (IQR) and comparisons between groups were made with Mann-Whitney U test. Differences between proportions were evaluated using chi-squared test. A p <0.05 (2-tailed tests) was considered significant.
Results
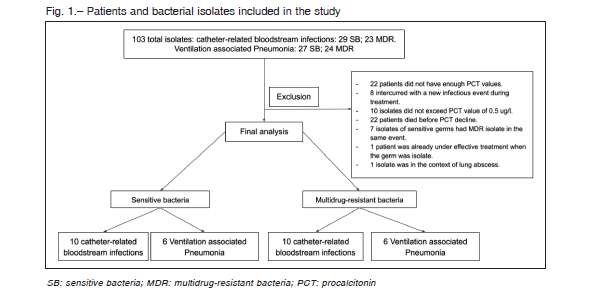
During the study period, bacterial isolates obtained from 103 patients were associated to VAP or CRBSI in ICU.
A total of 71 patients did not meet inclusion criteria (Figure 1). Finally, 16 patients with SB and 16 patients with MDR bacterial infections were selected for the analysis.
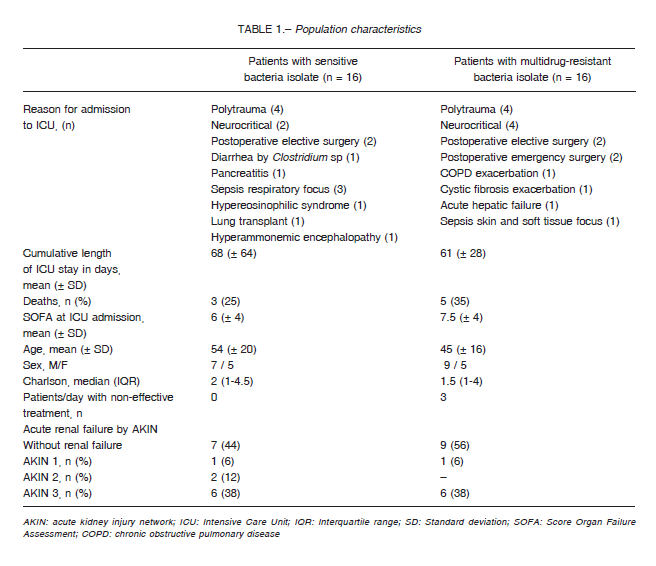
Patients with SB isolates had a mean age of 54 (SD ± 20) years and those with MDR isolates 45 (SD ± 16) years. Charlson median was 2 (IQR 1-4.5) in patients with SB infection and 1 (IQR 1-4) in patients with MDR infection.
Three patients with CRBSI were administered ineffective antibiotics for 48 hours: (a) in one patient initially treated with meropenem and colistin, ertapenem and tigecycline were added upon isolation of carbapenemresistant K. pneumoniae; (b) the second patient was empirically treated with vancomycin but upon isolation of vancomycin-resistant E. faecium the treatment was switched to linezolid; (c) the third patient was empirically treated with ceftriaxone and the antibiotic was changed to vancomycin upon isolation of methicillin-resistant S. epidermidis. These 6 treatment days represented 6% of the total days of antibiotics received by patients with MDR infections. The characteristics of the population are described in Table 1.
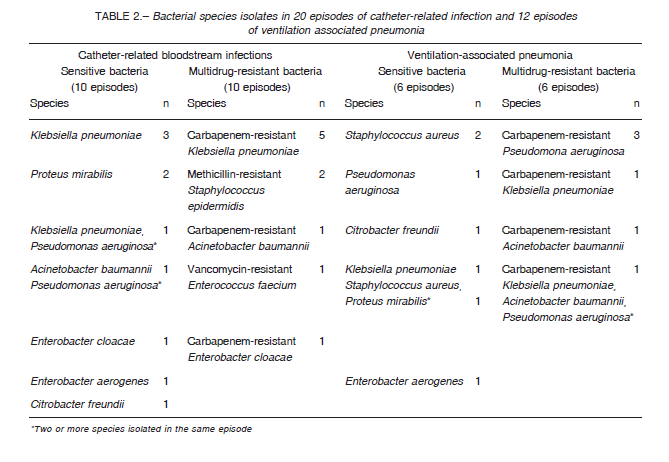
In patients with CRBSI secondary to SB, the species most frequently isolated was K. pneumoniae. In patients with MDR CRBSI, the germs most frequently isolated were carbapenemase-producing Enterobacteriaceae. In VAP generated by SB, the most frequent species was S. aureus whereas in VAP generated by MDR bacteria, the predominant germ was carbapenemase-producing P. aeruginosa (Table 2).
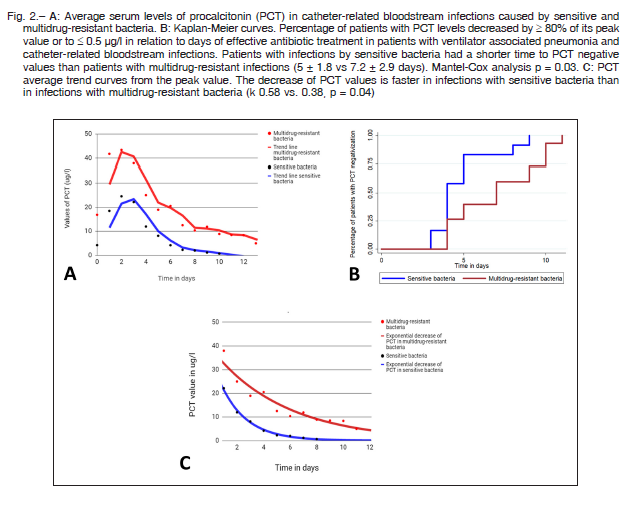
The maximum values of PCT in patients with CRBSI were significantly higher in MDR than in SB infections (mean ± SD 39 ± 30 μg/l vs. 10.7 ± 11, p = 0.02), Figure 2A. In VAP, patients with MDR infections had a mean ± SD PCT value of 3.2 ± 2 μg/l and patients with SB infections a mean ± SD PCT value of 5.0 ± 6 μg/l with no significant difference. The average time ± SD elapsed from the first sampling to the maximum PCT value was 24 ± 9 hours in MDR infections and 26±11 hours in SB infections. This difference was not statistically significant (Fig. 2).
The time elapsed from the detection of the bacteria to a decrease > 80% of the maximum PCT value or less than 0.5 μg/l was 7.2 ± 2.9 days in MDR vs. 5 ± 1.8 days in SB (p = 0.03) (Figure 2B). This analysis was carried out only with patients who received effective antibiotic therapy including both infections, VAP and CRBSI. As for the three patients who had initially received an ineffective antibiotic scheme, the analysis was started when an effective scheme was initiated. In both groups, the mean duration of antibiotic therapy was 13 ± 4.3 days, significantly longer than the 7.2 ± 2.9 days required for the decrease in PCT (p < 0.001). Changes in the decreasing tendency were not observed in 9 patients with SB infection and 7 patients with MDR infections who had acute kidney injury (Table 1).
The PCT decrease had an exponential trend line in both groups, responding to the following formula:
PCTt = PCT0 e–k t
PCTt: value of PCT at any time “t” (measured in days);
PCT0: value at the initial moment; k: time constant of the exponential; t: time measured in days.
The time constant k represents the velocity of PCT reduction. With higher k values, PCT will decline faster.
The k value for PCT was significantly lower in infections (VAP plus CRBSI) produced by MDR bacteria than in those produced by SB (mean ± SD 0.38 ± 0.15 1/days vs. 0.58 ± 0.2 1/days, p = 0.04), as shown in Figure 2C.
Discussion
Serum PCT levels were found to increase markedly between 6 to 12 hours after the onset of the inflammatory stimulus in sepsis, systemic infection, and severe inflammation21.
Also, elevated levels of PCT are correlated with the severity of the condition in humans and experimental animals 22.
We found that patients with CRBSI generated by MDR germs showed significantly higher PCT values than CRBSI generated by SB, reflecting a greater inflammatory response in MDR infections. This could be attributed to a greater virulence of MDR germs. Virulence and resistance are adaptive mechanisms in response to stressors such as host defence mechanisms and antibiotic pressure.
Plasmids are possibly the main vehicles for the transmission of genetic elements that encode virulence and resistance factors 23. This has been clearly described, for example, for plasmid pRSB107, located in certain E. coli strains, which expresses resistance to at least ten different antimicrobials and at the same time encodes four virulence factors 24. Carrying simultaneously plasmids that codify determinants of resistance and virulence enables the bacteria under antibiotic pressure to select the virulent ones and under conditions of infection, to select those resistant to antimicrobials 25.
In VAP, PCT values were similarly low in both groups, what agrees with the results of the meta-analysis by Hoeber et al., which associates bacteraemia with significantly higher values of PCT 26. However, we cannot reach any conclusions regarding this point because of the low number of VAP specimens.
We observed that PCT values decreased exponentially in both groups. In patients with SB infections, the decreasing constant of PCT was significantly higher than in MDR infections (a mean of 5 days in SB against 7.2 days in MDR). This might be ascribed to two causes. First, a higher virulence could trigger a more intense and prolonged inflammatory response in MDR infections. Second, the effective antibiotic treatment could be not as efficient against MDR organisms as are first-line antibiotics against sensitive bacteria. For example, carbapenems-resistant Enterobacteriaceae with MIC at meropenem ≥8-16 mcg/ml present a lower response to combined therapies with carbapenems compared to microorganisms with lower MIC levels 27, 28.
Additionally, the PCT decline occurred before the completion of antibiotic treatments in both MDR and SB infections (the mean duration of antibiotic therapy was 13 days in both groups). The time course of PCT provides information on the resolution of severe bacterial infections 14.
We observed in our study that the duration of the antibiotic treatment may be reduced by PCT-guided protocols, even in infections caused by MDR bacteria. However, this was just a retrospective observational study and further studies are necessary to determine the safety of PCT-guided protocols in MDR infections.
The main limitation of our study is the low size of the sample. Indeed, 69% of the patients did not meet the inclusion criteria. Our primary objective was a descriptive study of PCT kinetics, therefore we only included patients with complete PCT curves.
In sum, we found that the inflammatory response measured by PCT was stronger and more prolonged in MDR bacteraemia, which may be ascribed to a higher virulence of MDR bacteria. However, even in MDR infections, the decline of PCT levels occurred well before completion of antibiotic courses. Therefore, it is necessary to study the potential application of antibiotic protocols guided by PCT in MDR infections to reduce the antibiotics exposure in this population.
Conflicts of interest: None to declare
References
1. Crémieux A-C, Muller-Serieys C, Panhard X, et al. Emergence of resistance in normal human aerobic commensal flora during telithromycin and amoxicillin-clavulanic acid treatments. Antimicrob Agents Chemother 2003; 47: 2030-5.
2. Lodise TP, Miller CD, Graves J, et al. Clinical prediction tool to identify patients with Pseudomonas aeruginosa respiratory tract infections at greatest risk for multidrug resistance. Antimicrob Agents Chemother 2006; 51: 417-22.
3. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096.
4. Llewelyn MJ, Fitzpatrick JM, Darwin E, et al. The antibiotic course has had its day. BMJ 2017; j3418.
5. Peterson LR. Bad bugs, no drugs: No ESCAPE revisited. Clin Infect Dis 2009; 49: 992-3.
6. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63: 194-200.
7. Hecker MT, Aron DC, Patel NP, et al. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003; 163: 972-8.
8. Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care 2013; 17: R202.
9. Lossa GR, Giordano Lerena R, Fernández LE, et al. Prevalencia de infecciones hospitalarias en unidades de cuidados intensivos para adultos en Argentina. Rev Panam Salud Pública 2008; 24: 324–30.
10. Goldmann DA. Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms in hospitals. A challenge to hospital leadership. JAMA 1996; 275: 234-40.
11. Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis 2017; 4: ofw249.
12. Bouadma L, Luyt C-E, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375: 463-74.
13. Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med 2014; 190: 1102-10.
14. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16: 819-27.
15. Bloos F, Trips E, Nierhaus A, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med 2016; 176: 1266-76.
16. Vallés J, Alvarez-Lerma F, Palomar M, et al. Health-careassociated bloodstream infections at admission to the ICU. Chest 2011; 139: 810-5.
17. Koulenti D, Lisboa T, Brun-Buisson C, et al. Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med 2009; 37: 2360-9.
18. da Silva Winter J, dos Santos RP, de Azambuja AZ, et al. Microbiologic isolates and risk factors associated with antimicrobial resistance in patients admitted to the intensive care unit in a tertiary care hospital. Am J Infect Control 2013; 41: 846-8.
19. Shrivastava S, Shrivastava P, Ramasamy J. World Health Organization releases global priority list of antibioticresistant bacteria to guide research, discovery, and development of new antibiotics. Journal of Medical Society 2018; 32: 76.
19. Vallés J, Alvarez-Lerma F, Palomar M, et al. Health-careassociated bloodstream infections at admission to the ICU. Chest 2011; 139: 810-5.
20. Kellum JA, Levin N, Bouman C, et al. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care 2002; 8: 509-14.
21. Müller B, Becker KL. Procalcitonin: how a hormone became a marker and mediator of sepsis. Swiss Med Wkly 2001; 131: 595-602.
22. Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med 2008; 36: 941-52.
23. Beceiro A, Tomás M, Bou G. Resistencia a los antimicrobianos y virulencia, ¿una asociación beneficiosa para el mundo microbiano? Enferm Infecc Microbiol Clin 2012; 30: 492-9.
24. Szczepanowski R, Braun S, Riedel V, et al. The 120 592 bp IncF plasmid pRSB107 isolated from a sewage-treatment plant encodes nine different antibiotic-resistance determinants, two iron-acquisition systems and other putative virulence-associated functions. Microbiology 2005; 151: 1095-111.
25. Martínez JL, Baquero F. Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin Microbiol Rev 2002; 15: 647-79.
26. Hoeboer SH, van der Geest PJ, Nieboer D, et al. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015; 21: 474-81.
27. Bratu S, Tolaney P, Karumudi U, et al. Carbapenemaseproducing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother 2005; 56: 128-32.
28. Pournaras S, Vrioni G, Neou E, et al. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int J Antimicrob Agents 2011; 37: 244-7.
[…] Objectivity cannot be equated with mental blankness; rather, objectivity resides in recognizing your preferences and then subjecting them to especially harsh scrutiny– and also in a willingness to revise or abandon your theories when the tests fail (as they usually do).
[…] La objetividad no puede igualarse a una mente en blanco, más bien reside en reconocer vuestras preferencias y someterlas a un duro escrutinio– y también a una buena voluntad de revisar y abandonar sus teorías cuando las pruebas fallan (cómo usualmente ocurre).
Stephen Jay Gould (1941-2002)
Capturing the Center. Natural History 107 (December 1998): 18
