*en inglés
ROMINA J. FERNÁNDEZ-BRANDO ¹, MARÍA MARTA AMARAL ², ANDRÉS E. CIOCCHINI ³, LETICIA V. BENTANCOR 4, JORGE A. TRELLES 5, MARCELO DA ROCHA 6, MARTÍN LANDRIEL 7, MARIANA UGARTE 7, GABRIEL BRIONES ³, CRISTINA IBARRA ²*, MARINA S. PALERMO ¹*
¹ Laboratorio de Patogénesis y de Procesos Infecciosos, Instituto de Medicina Experimental, (IMEX-CONICET), Academia Nacional de Medicina, ² Laboratorio de Fisiopatogenia, Departamento de Fisiología, Instituto de Fisiología y Biofísica Bernardo Houssay (IFIBIO Houssay-CONICET), Facultad de Medicina, Universidad de Buenos Aires, ³ Instituto de Investigaciones Biotecnológicas Rodolfo Ugalde-Instituto Tecnológico de Chascomús (IIB-INTECH), Universidad Nacional de San Martín (UNSAM), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), 4 Laboratorio de Ingeniería Genética y Biología Celular y Molecular, Universidad Nacional de Quilmes, 5 Laboratorio de Investigaciones en Biotecnología Sustentable (LIBioS), Departamento de Ciencia y Tecnología, Universidad Nacional de Quilmes, 6Asociación Lucha contra el Síndrome Urémico Hemolítico (LuSUH), 7 Cátedra de Nutrición, Carrera de Tecnología de Alimentos, Universidad Nacional de Lanús, Buenos Aires, Argentina
*Authors contributed equally to this work
Resumen Control microbiológico y serológico de Escherichia coli O157: H7 en personal de jardines de infantes de la ciudad de Buenos Aires y áreas suburbanas. Las infecciones bacterianas con Escherichia coli productor de toxina Shiga (Stx) (STEC) están implicadas en el desarrollo del síndrome urémico hemolítico (SUH). A pesar de la magnitud del problema social y económico causado por el SUH, actualmente no existe un tratamiento específico o una vacuna eficaz para uso humano. Por lo tanto, la prevención de las infecciones por STEC es la tarea central para reducir la incidencia del SUH. Esto es especialmente cierto para Argentina en donde el SUH muestra un comportamiento endémico y presenta una incidencia extremadamente alta entre los niños. En efecto, la mediana de casos notificados en menores de 5 años para el periodo 2010-2015 fue 306, mientras que la tasa de notificación fue 8.5 casos cada 100 000 menores/año (http://www.msal.gob.ar/images/stories/boletines/boletin_integrado_vigilancia_N335-SE45.pdf). El objetivo de este trabajo fue analizar serológicamente al personal adulto de jardines de infantes de la ciudad de Buenos Aires y el área suburbana con el fin de detectar portadores, y brindarles formación sobre las buenas prácticas para reducir la transmisión de infecciones con STEC y así evitar el SUH. También se evaluó la calidad microbiológica de las muestras de agua y de la comida elaborada en los mismos jardines. Hemos estudiado 67 adultos, a través del hisopado de manos para la búsqueda de STEC y suero para la presencia de anticuerpos contra Stx y el lipopolisacárido (LPS) de serogrupo O157. También se analizaron 13 suministros de agua y 6 muestras de comida pertenecientes a 6 jardines de infantes públicos. Se identificaron 46 individuos positivos para Stx2, pero solo 7 para LPS-O157. No se detectó presencia de patógenos STEC en las muestras de las manos del personal, ni en los reservorios de agua o muestras de comida.
Palabras clave: síndrome urémico hemolítico, anticuerpos anti-Stx; anti-LPS O157, charlas de promoción de la salud, cajas didácticas
Abstract Shiga toxin (Stx)-producing Escherichia coli (STEC) infections are implicated in the development of the life-threatening hemolytic-uremic syndrome (HUS). Despite the magnitude of the social and economic problems caused by HUS, no licensed vaccine or effective therapy is currently available for human use. Prevention of STEC infections continues being the most important measure to reduce HUS incidence. This is especially true for Argentina where HUS incidence among children is extremely high and shows an endemic pattern. The aim of this work was to investigate serologically adult staff of kindergartens in Buenos Aires city and suburban areas in order to detect possible carriers, and to educate personnel about good practices to reduce HUS transmission. We also assessed the microbiological quality of water and meal samples from the same kindergartens. We tested 67 healthy adults, 13 water supplies and 6 meals belonging to 6 public kindergartens. We analysed hand swabs for isolation of STEC and serum samples for the presence of antibodies against Stx and lipopolysaccharide (LPS) of O157 serogroup. We identified 46 Stx2-positive individuals, but only 7 for O157 LPS. No presence of STEC pathogens was detected in hands of staff, water or meal samples.
Key words: hemolytic uremic syndrome, anti-Stx antibodies, anti-O157 LPS, health promotion talks, teaching boxes
Received: 23/10/16 Accepted: 10/1/17
Postal address: Dr. Marina S. Palermo, Instituto de Medicina Experimental (IMEX-CONICET), Academia Nacional de Medicina, Pacheco de Melo 3081, 1425 Buenos Aires, Argentina
Pathogenic Shiga toxin (Stx)-producing Escherichia coli (STEC) infections can cause illness with a wide spectrum of severity, from watery diarrhea and hemorrhagic colitis to hemolytic uremic syndrome (HUS), a life-threatening condition1. The infection correlates with the ingestion of contaminated meat or vegetables, but is also transmitted by water or even person-to-person contact2, 3. Sporadic or massive outbreaks have been reported in several developed countries4, but in Argentina, HUS shows an endemic pattern and represents a serious public health problem with high morbidity and mortality rates5-7. Escherichia coli O157:H7 is the dominant STEC serotype associated with HUS worldwide and also in Argentina, although non-O157 STEC serogroups can cause a similar disease.
A striking feature of STEC infections is the production of potent Shiga toxins (Stx), responsible for HUS development8, 9. STEC can produce two types of Stx, type 1 (Stx1) and type 2 (Stx2), and their allelic variants, all of them have an AB5 molecular structure10, 11.
Children below 5-years old are the most susceptible population to develop STEC-associated HUS, and attending to child-care or kindergarten institutions have been reported to be a risk for HUS12-15, probably as a consequence of person-to-person infection route.
Thus, the aim of the present study was to analyze the frequency of asymptomatic STEC carriers on kindergarten’s staff, by testing anti-O157LPS and anti-Stx antibodies.
Laboratory diagnosis of STEC O157 infections relies on isolation of the pathogen from stools6, 7, detection of Stx in fecal filtrates16, and/or detection of anti-Stx serum antibodies17. The detection of anti-O157 E. coli lipopolysaccharide (LPS) antibodies in combination with stool culture and/or serum Stx antibodies by western blot, considerably improves the diagnosis of STEC infections, mainly in those patients in which bacteria were not isolated18-20 or in asymptomatic adults.
Although several methods have been used to test the presence of anti-Stx antibodies, i.e. neutralization assay (Stx-Nab) and enzyme-linked immunosorbent assays (ELISA)17, we chose western blot (WB) because it proved to be more specific and sensitive than ELISA21, 22. As an additional advantage, WB allows to discriminate antibodies against A and B subunits.
In addition, we analysed the microbiological quality of water and meals and hand-swab samples of kindergarten staff.
At the same time, we informed the staff on health risks and provided them with the necessary tools to avoid those risks, including information on food preparation and appropriate hygiene practices to prevent diarrhea diseases, particularly associated to STEC pathogens.
Due to the high incidence of HUS in Argentina and the lack of a licensed vaccine or specific and effective therapy, the primary prevention is fundamental to decrease the impact of HUS in children’s health.
Materials and methods
Six public kindergartens situated in Buenos Aires city and suburban areas were studied between November 21, 2012 and May 21, 2013. The Ethics Committee of the University of Buenos Aires approved this study. All volunteers were enrolled in this study after the written informed consent had been obtained.
Several health promotion talks were given to the kindergartens’ staff. The information provided in the talks included adequate food handling and hygiene practices to prevent diarrhea. In this regard, the kindergartens’ staff were trained through theoretical talks and teaching boxes created by the LUSUH Association (Bulletin N° 15, www.lusuh.org.ar) containing puppets and scripts in order to develop three plays for children on the following topics: washing hands, washing fruits and vegetables, and proper cooking of meat; as well as warning signs to place in kitchens and bathrooms (www.lusuh.org.ar). In addition, a video explaining the characteristics of the disease was shown and a book of testimonials from parents of children who have suffered from HUS was given.
Sixty-seven healthy adults belonging to the kindergartens’ staff were analyzed. Blood samples were obtained by pricking the index finger with a needle and collecting the blood with heparinized capillary tubes. Plasma was immediately separated and stored at -20 °C until use. Hand-swab samples of kindergarten staff were simultaneously obtained.
Water samples (200 ml each sample) were obtained in sterilized tubes from different sources (water tank, kitchen tap, bathroom tap). Prior to sample collection, the sources were sterilized by alcohol and flamed. All samples were stored at 4 °C. Three out of six kindergartens served food prepared in place, the rest of them served snacks such as crackers, pastries and / or bread, with milk (packaged in plastic sachets), mate or tea infusions. Because the collations do not represent a risk for STEC transmission, only kindergartens in which food was prepared were included for food sampling. The food analyzed was: bread and butter; breaded beef; boiled chicken; boiled potato, carrot and pumpkin; noodles and cornstarch and milk based dessert. All food samples were kept refrigerated until the microbiological analysis was done.
Water samples were filtered using a sterilized filter. Filters were incubated in LB broth to increase the bacterial concentration in the sample. Samples were plated on MacConkey-sorbitol and were incubated for 24 h at 37 °C. Presence or absence of transparent/clear colonies (nonsorbitol-fermenting) was observed. Sixty five grams of each food sample were studied for the presence of O157:H7 E. coli strains following the BAM 2011 methodology (http://www.fda.gov/Food/FoodScienceResearch/ LaboratoryMethods/ucm070080.htm).
Hand swabs of 30 healthy adults from kindergartens’ staff were recorded and analyzed. The swabs were inoculated in Stuart medium tubes (Difco Laboratories, Detroit, MI, USA), ice-cooled, and analyzed within 24 h. Samples were placed on MacConkey-sorbitol and incubated for 24 h at 37 °C. Presence or absence of colonies was observed.
Purified Stx2 and Stx1 toxins were produced and stored at -20 °C until its usage in WB assay, following previously reported methods22. WB assay was adapted from the method of Karmali et al.23, as previously described22.
A novel indirect ELISA test for the diagnosis of STEC-associated diseases has been developed recently20. It is based on a recombinant glycoprotein consisting of an N-formylperosamine O polysaccharide attached to the C. jejuni AcrA protein. The lack of reactivity against non-glycosilated form of AcrA and the lack of cross-reactivity among different glycoproteins confirm the specificity of the assay. Microtiter plates (Corning) were coated with 50 μl containing 2.5 g/ml of O157-AcrA (125 ng/well), as previously described20. Positive and negative control samples were included in each plate. Subsequently, bound antibodies were detected using horseradish peroxidase (HRP)-conjugated goat antibodies anti-human total Ig (Sigma). The optimal sample dilution was 1:100, and the optimal dilution of conjugates was 1:2 000. These established parameters20 were used to test all samples.
Results were expressed as the percentage of reactivity of the mean absorbance at 450 nm (A450) of the positive-control serum included in each assay run. The percentage of reactivity was calculated as follows: % reactivity (A450 of the test sample/mean A450 of the positive control) × 100.
Frequency values were compared with the Chi-square test for independence and trend (two by three tables) or Fisher’s exact test (two by two tables) as indicated.
Results
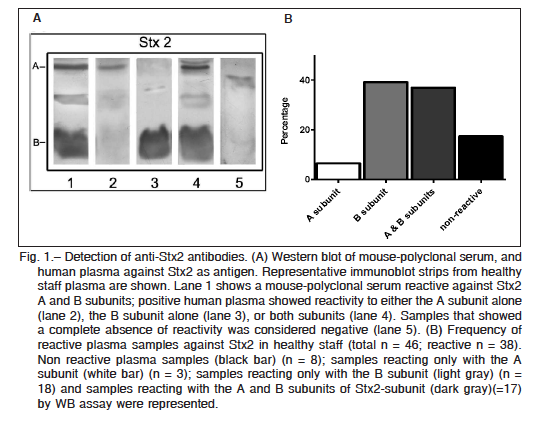
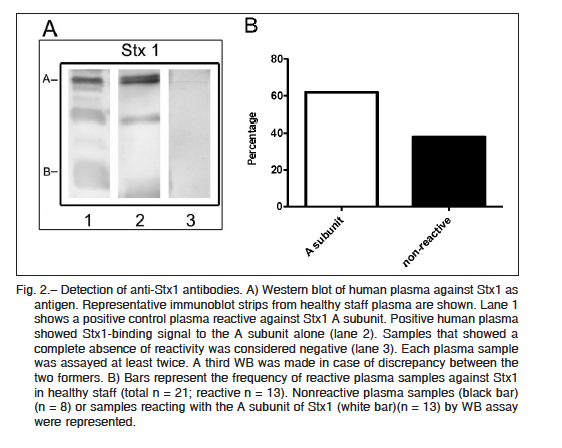
Purified Stx2 and Stx1 were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and after transferring it onto polyvinylidene difluoride (PVDF) membranes, two bands corresponding to the molecular mass of A and B subunits of each toxin were identified with either anti-Stx2 mouse polyclonal antibodies or anti-Stx1 positive human plasma (Fig. 1A and Fig. 2A). Thirty eight (out of 46) plasma samples were reactive at least against one subunit of Stx2, indicating that 83% of adults were positive for Stx2 (Fig. 1B). These samples were reactive with either the A subunit alone (n = 3), the B subunit alone (n = 18), or both subunits (n = 17), while only 8 samples were negative for Stx2. In contrast to the high frequency of Stx2-positive samples, only 13 plasma samples out of 21 were positive for Stx1 (62%). All Stx1 positive samples were reactive only with the A subunit (Fig. 2B). Given that A subunits from Stx1 and Stx2 show a high homology in their sequences (36) we cannot rule out that antibodies against Stx2 A subunit cross-reacted with Stx1 A subunit.
Since one of the most immunodominant STEC antigens is the O-polysaccharide section of the LPS and the O157 serogroup is the most prevalent among HUS cases in Argentina, we use the recently developed anti- LPS O157-AcrA ELISA test20, because it is a simple, sensitive and specific test to search for asymptomatic adults as possible carriers of O157-STEC infection.
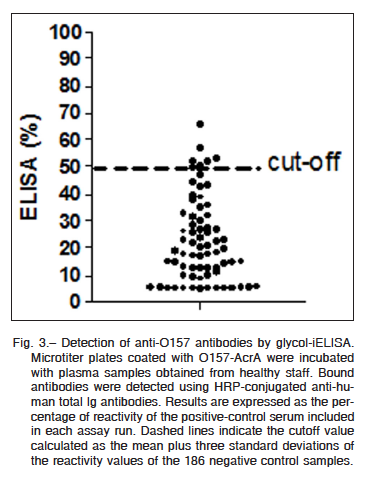
All 62 serum samples were analyzed and the results were outlined in a dot plot diagram (Fig. 3). Based on a previous work20, a cut-off value was established as the mean plus three standard deviations of the reactivity values of negative control samples. Over this cut-off it is possible to clearly discriminate between negative and positive anti-O157 samples. As shown in Fig. 3 specific reactivity against O157-AcrA was observed in seven samples. The detected antibody response was specifically directed toward the O-polysaccharide moiety of the corresponding glycoprotein in all samples (data not shown).
Food samples included bread and butter, breaded beef, chicken, noodles, boiled carrots and potatoes, mashed pumpkin and a dessert made of cornstarch and milk. All food samples analyzed according to FDA – BAM 2011 were negative for E coli O157: H7. Similarly, water supplies and hand swabs cultures did not show growth of pathogenic E. coli strains.
Discussion
E. coli O157 serogroup, specifically Stx2a/c-producing strains, are the most virulent diarrhegenic E. coli known to date24. Because of the extremely high incidence of HUS in children below 5-year-old in Argentina, STEC infections in childcare facilities and kindergartens are a public health problem that requires the improvement of surveillance policies. Although national health organisms have significantly improved national surveillance for waterborne and foodborne diseases during the last decade, no national surveillance exists for STEC-associated diseases resulting primarily from person-to-person transmission.
Several epidemiological studies have reported STEC-associated diarrheal outbreaks in child-care centers12-15, 25, and attending to these institutions is considered a risk for HUS development6, 24. Considering that in all these outbreaks no common source of infection was found, and that secondary cases were identified as a part of the epidemic curve, person-to-person transmission has been suspected. Major recommendations about attendant children include: i) requirements to keep symptomatic children at home, ii) early case reporting, and iii) exclusion of those who remain culture-positive. But also symptomatic or asymptomatic adults have been focus of attention. Thus, staff members of child-care centers, kindergartens and close adult family contacts have been studied. Worldwide recommendation is that all staff member positive for STEC should be excluded from the center until microbiological clearance is obtained. Other environmental factors that were identified as contributors to spread of diarrhea illness included overcrowding and inadequacies in cleaning and in the food preparation facilities26. Moreover, reports demonstrating diarrheal illness in a family member as a risk factor for developing HUS in Canada also support person-to-person transmission27-29.
Prevention and control of person-to-person STEC transmission depend primarily on appropriate hands hygiene, environmental hygienic conditions, appropriate water suppliers30, but also on an appropriate level of risk awareness by the staff.
In spite of the high circulation of STEC strains in Argentina, in particular those producing Stx27, local information about the frequencies of healthy STEC carriers is very limited. This is due, at least in part, to the lack of microbiological evaluation of those individuals. We have sorted this difficulty screening anti-O157 LPS antibodies in plasma from kindergartens’ staff, by a simple, specific and economical ELISA test. We studied 67 adults from kindergartens’ staff and detected 83% of Stx2-positive plasma samples using the same methodology by which we previously reported 67% of healthy children showing anti Stx2-antibodies22. These results suggest that primary STEC infection mainly occurred during childhood, and post-bacterial contacts ensure the persistence of anti-Stx2 antibodies. On the other hand, this frequency was significantly higher than the frequencies reported for urban areas at Canada (46%) but similar to those of a rural area (65%) for an age-matched population23, probably due to the high circulation of Stx2-producing strains in Argentina and associated to the HUS endemic behavior in this country6, 7.
A previous study has found 51% of HUS household contacts with neutralizing anti-Stx2 antibodies in Argentina31. The difference between both studies can be ascribed to the different sensitivity of the tests used, i.e. western blot vs. neutralizing VERO assay, rather than to a real increase in adult seropositivity. Indeed, STEC-positive adults or children are often detected when a household child undergoes STEC-associated bloody diarrhea or HUS32. The percentage of anti-Stx1 positive plasma among staff was significantly lower (62%) than the percentage of anti-Stx2 (83%), but still higher compared to the previous study in children from Argentina (<10%)20. Moreover, these frequencies are higher to those found in the healthy populations from Canada23, Europe33 and the United States34. Considering that all anti-Stx1- positive samples were only reactive against the A subunit , it might be possible that antibodies against the A subunit of Stx1 resulted from cross-reaction with A subunit of Stx2, based on their known antigenic similarities35. It is known the high prevalence of Stx2a/Stx2c-producing STEC in beef cattle36, as well as in stools from post-enteric HUS cases in Argentina9. However, since all negative samples for Stx1 reacted with Stx2 A subunit, it is difficult to ascribed anti-Stx1 positivity only to the cross-reactivity with Stx2. Because anti-Stx antibodies probably do not prevent re-infections with STEC, the existence of a large asymptomatic population of adults that carries the pathogenic strains without systemic complications is probable. In fact, the identification of 11% of positive samples reactive against O157 LPS suggests that they are probably carrying or were recently infected with these pathogenic bacteria. It is important to highlight that several authors have reported that the median duration of O157-STEC shedding was 31 days, and median period of exclusion was 39.5 days14.
Water supply, food and hand swabs for isolation of STEC pathogens showed negative results, suggesting that personal hygiene and water and food suppliers are a common problem. However, we could not completely rule out a failure at the level of sample preparation, or that bacterial concentration in samples was below the sensitivity of the test used37.
In conclusion, despite the low number of adults under presumption of being STEC carriers, the risk of transmission of these pathogens to children in close contact with them, makes education a central focus of interest. In fact, specific education in prevention of STEC dissemination toward adult staff at kindergartens is essential to ensure good practices in child stool handling, hand washing, and exclusion of attendees (children and staff) with diarrhea. To help control of STEC infections, the education of staff working in kindergartens can improve habits of staff in close contact with children at the age of high-risk of developing HUS. In addition, it is important to highlight that although we focus staff education just on STEC transmission, it will certainly result in the improvement of surveillance for acute gastrointestinal diseases by various other (viruses, Shigella) also associated to person-to-person transmission.
Acknowledgements: We thank school authorities and educational staff for their excellent collaborative attitude. This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (grant number PICTO-CIN 2010/0077). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interests: None to declare
References
1. Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol 2004; 2: 123-40.
2. Caprioli A, Morabito S, Brugere H, Oswald E. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet Res 2005; 36: 289-311.
3. Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev 1991; 13: 60-98.
4. Böhnlein C, Kabisch J, Meske D, Franz CM, Pichner R. Fitness of EHEC/EAEC O104:H4 in comparison to EHEC O157: survival studies in food and in vitro. Appl Environ Microbiol 2016; 82: 6326-34.
5. Lopez EL, Prado-Jimenez V, O’Ryan-Gallardo M, Contrini MM. Shigella and Shiga toxin-producing Escherichia coli causing bloody diarrhea in Latin America. Infect Dis Clin North Am 2000; 14: 41-65 viii.
6. Rivas M, Miliwebsky E, Chinen I, Deza N, Leotta GA. Epidemiología del Síndrome Urémico Hemolítico en Argentina. Diagnóstico del agente etiológico, reservorios y vías de transmisión. Medicina (B Aires) 2006; 66 (Suppl 3): 27-32.
7. Rivas M, Miliwebsky E, Chinen I, et al. Characterization and epidemiologic subtyping of Shiga toxin-producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodborne Pathog Dis 2006; 3: 88-96.
8. Noel J M, Boedeker EC. Enterohemorrhagic Escherichia coli: a family of emerging pathogens. Dig Dis 1997;15: 67-91.
9. O’Brien AD, Tesh VL, Donohue-Rolfe A, et al. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr Top Microbiol Immunol 1992;180: 65-94.
10. Stein PE, Boodhoo A, Tyrrell GJ, Brunton JL, Read RJ. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature 1992; 355: 748-50.
11. Fraser ME, Chernaia MM, Kozlov YV, James MN. Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 A resolution. Nat Struct Biol 1994; 1: 59-64.
12. Raffaelli RM, Paladini M, Hanson H, et al. Child care-associated outbreak of Escherichia coli O157:H7 and hemolytic uremic syndrome. Pediatr Infect Dis J 2007; 26: 951-3.
13. Gilbert M, Monk C, Wang HL, Diplock K, Landry L. Screening policies for daycare attendees: lessons learned from an outbreak of E. coli O157:H7 in a daycare in Waterloo, Ontario. Can J Public Health 2008; 99: 281-5.
14. Dabke G, Le Menach A, Black A, et al. Duration of shedding of Verocytotoxin-producing Escherichia coli in children and risk of transmission in childcare facilities in England. Epidemiol Infect 2014; 142: 327-34.
15. Gomez D, Miliwebsky E, Silva A, et al. Aislamiento de Escherichia coli productor de toxina Shiga durante un brote de gastroenteritis en un Jardín Maternal de la Ciudad de Mar del Plata. Rev Argent Microbiol 2005; 37: 176-83.
16. Yamada S, Matsushita S, Kai A, et al. Detection of verocytotoxin from stool and serological testing of patients with diarrhea caused by Escherichia coli O157 : H7. Microbiol Immunol 1993; 37: 111-8.
17. Reymond D, Karmali MA, Clarke I, Winkler M, Petric M. Comparison of the western blot assay with the neutralizing-antibody and enzyme-linked immunosorbent assays for measuring antibody to verocytotoxin 1. J Clin Microbiol 1997;35: 609-13.
18. Kulkarni H, Goldwater PN, Martin A, Bettelheim KA. Escherichia coli ‘O’ group serological responses and clinical correlations in epidemic HUS patients. Comp Immunol Microbiol Infect Dis 2002; 25: 249-68.
19. Bitzan M, Moebius E, Ludwig K, et al. High incidence of serum antibodies to Escherichia coli O157 lipopolysaccharide in children with hemolytic-uremic syndrome. J Pediatr 1991; 119: 380-5.
20. Melli LJ, Ciocchini AE, Caillava AJ, et al. Serogroup-specific bacterial engineered glycoproteins as novel antigenic targets for diagnosis of Shiga toxin-producing-escherichia coli-associated hemolytic-uremic syndrome. J Clin Microbiol. 2015; 53: 528-538.
21. Ludwig K, Karmali MA, Sarkim V, et al. Antibody response to Shiga toxins Stx2 and Stx1 in children with enteropathic hemolytic-uremic syndrome. J Clin Microbiol 2001; 39: 2272-9.
22. Fernández-Brando RJ, Bentancor LV, Mejías MP, et al. Antibody response to Shiga toxins in Argentinean children with enteropathic hemolytic uremic syndrome at acute and long-term follow-up periods. PLoS One 2011; 29; 6(4): e19136.
23. Karmali MA, Mascarenhas M, Petric M, et al. Age-specific frequencies of antibodies to Escherichia coli verocytotoxins (Shiga toxins) 1 and 2 among urban and rural populations in southern Ontario. J Infect Dis 2003; 188: 1724-9.
24. Rivas M, Chinen I, Miliwebsky E, Masana M. Risk Factors for Shiga Toxin-Producing Escherichia coli-Associated Human Diseases. Microbiol Spectr 2014; 2(5). doi: 10.1128/microbiolspec.EHEC-0002-2013
25. O’Donnell JM, Thornton L, McNamara EB, Prendergast T, Igoe D, Cosgrove C. Outbreak of Vero cytotoxin-producing Escherichia coli O157 in a child day care facility. Commun Dis Public Health 2002; 5: 54-8.
26. Busani L, Boccia D, Caprioli A, et al. Public health implications of a case of haemolytic-uraemic syndrome associated with a concomitant outbreak of mild gastroenteritis in a small rural community. Epidemiol Infect 2006; 134: 407-13.
27. Ludwig K, Sarkim V, Bitzan M, et al. Shiga toxin-producing Escherichia coli infection and antibodies against Stx2 and Stx1 in household contacts of children with enteropathic hemolytic-uremic syndrome. J Clin Microbiol 2002; 40: 1773-82.
28. Rowe PC, Orrbine E, Lior H, Wells GA, McLaine PN. Diarrhoea in close contacts as a risk factor for childhood haemolytic-uraemic syndrome. The CPKDRC co-investigators. Epidemiol Infect 1993; 110: 9-16.
29. Rowe PC, Orrbine E, Ogborn M, et al. Epidemic Escherichia coli O157:H7 gastroenteritis and hemolytic-uremic syndrome in a Canadian Inuit community: intestinal illness in family members as a risk factor. J Pediatr 1994; 124:21-6.
30. McCall BJ, Slinko VG, Smith HV, et al. An outbreak of shiga toxin-producing Escherichia coli infection associated with a school camp. Commun Dis Intell Q Rep 2010; 34: 54-6.
31. Lopez EL, Diaz M, Devoto S, et al. Evidence of infection with organisms producing Shiga-like toxins in household contacts of children with the hemolytic uremic syndrome. Pediatr Infect Dis J 1991; 10: 20-4.
32. Werber D, Mason BW, Evans MR, Salmon RL. Preventing household transmission of Shiga toxin-producing Escherichia coli O157 infection: promptly separating siblings might be the key. Clin Infect Dis 2008; 46: 1189-96.
33. Bitzan M, Ludwig K, Klemt M, et al. The role of Escherichia coli O 157 infections in the classical (enteropathic) haemolytic-uraemic syndrome: results of a Central European, multicentre study. Epidemiol Infect 1993; 110: 183-96.
34. Barrett TJ, Green JH, Griffin PM, et al. Enzyme linkedimmunosorbent assays for detecting antibodies to Shiga-like toxin I, Shiga-like toxin II, and Escherichia coli O157:H7 lipopolysaccharide in human serum. Curr Microbiol 1991; 23: 189-95.
35. Beddoe T, Paton AW, Le Nours J, Rossjohn J, Paton JC. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci 2010; 35: 411-8.
36. Masana MO, Leotta GA, Del Castillo LL, et al. Prevalence, characterization, and genotypic analysis of Escherichia coli O157:H7/NM from selected beef exporting abattoirs of Argentina. J Food Prot 2010; 73: 649-56.
37. Kim SR, Yoon Y, Kim WI, et al. Comparison of sample preparation methods for the recovery of foodborne pathogens from fresh produce. J Food Prot 2012; 75: 1213-8.
